Chemistry:Nitrate
| |
| Names | |
|---|---|
| Systematic IUPAC name
Nitrate | |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| NO−3 | |
| Molar mass | 62.004 g·mol−1 |
| Conjugate acid | Nitric acid |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Nitrate is a polyatomic ion with the chemical formula NO−3. Salts containing this ion are called nitrates. Nitrates are common components of fertilizers and explosives.[1] Almost all inorganic nitrates are soluble in water. An example of an insoluble nitrate is bismuth oxynitrate.
Structure
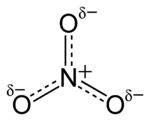
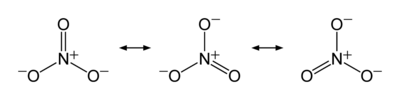
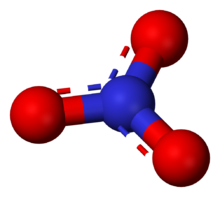
The ion is the conjugate base of nitric acid, consisting of one central nitrogen atom surrounded by three identically bonded oxygen atoms in a trigonal planar arrangement. The nitrate ion carries a formal charge of −1. This charge results from a combination formal charge in which each of the three oxygens carries a −2⁄3 charge, whereas the nitrogen carries a +1 charge, all these adding up to formal charge of the polyatomic nitrate ion. This arrangement is commonly used as an example of resonance. Like the isoelectronic carbonate ion, the nitrate ion can be represented by resonance structures:
Dietary nitrate
A rich source of inorganic nitrate in the human diets come from leafy green foods, such as spinach and arugula. NO−3 (inorganic nitrate) is the viable active component within beetroot juice and other vegetables. Drinking water is also a dietary source.[2]
Dietary nitrate supplementation delivers positive results when testing endurance exercise performance.[3]
Ingestion of large doses of nitrate either in the form of pure sodium nitrate or beetroot juice in young healthy individuals rapidly increases plasma nitrate concentration by a factor of 2 to 3, and this elevated nitrate concentration can be maintained for at least 2 weeks. Increased plasma nitrate stimulates the production of nitric oxide, NO. Nitric oxide is an important physiological signaling molecule that is used in, among other things, regulation of muscle blood flow and mitochondrial respiration.[4]
Cured meats
Nitrite consumption is primarily determined by the amount of processed meats eaten, and the concentration of nitrates in these meats. Although nitrites are the nitrogen compound chiefly used in meat curing, nitrates are used as well. Nitrates lead to the formation of nitrosamines.[5] The production of carcinogenic nitrosamines may be inhibited by the use of the antioxidants vitamin C and the alpha-tocopherol form of vitamin E during curing.[6]
Many meat processors claim their meats (e.g. bacon) is "uncured" - which is a marketing claim with no factual basis: there is no such thing as "uncured" bacon (as that would be, essentially, raw sliced pork belly).[7][better source needed] "Uncured" meat is in fact actually cured with nitrites with virtually no distinction in process -- the only difference being the USDA labeling requirement between nitrite of vegetable origin (such as from celery) vs. 'synthetic' sodium nitrite. (An analogy would be purified "sea salt" vs. sodium chloride - both being the exact same chemical with the only essential difference being the origin.)
Anti-hypertensive diets, such as the DASH diet, typically contain high levels of nitrates, which are first reduced to nitrite in the saliva, as detected in saliva testing, prior to forming nitric oxide.[2]
Occurrence and production
Nitrate salts are found naturally on earth in arid environments as large deposits, particularly of nitratine, a major source of sodium nitrate.
Nitrates are produced by a number of species of nitrifying bacteria in the natural environment using ammonia or urea as a source of nitrogen and source of free energy. Nitrate compounds for gunpowder were historically produced, in the absence of mineral nitrate sources, by means of various fermentation processes using urine and dung.
Lightning strikes in earth's nitrogen- and oxygen-rich atmosphere produce a mixture of oxides of nitrogen, which form nitrous ions and nitrate ions, which are washed from the atmosphere by rain or in occult deposition.
Nitrates are produced industrially from nitric acid.[1]
Uses
Agriculture
Nitrates are used as fertilizers in agriculture because of their high solubility and biodegradability. The main nitrate fertilizers are ammonium, sodium, potassium, calcium, and magnesium salts. Several billion kilograms are produced annually for this purpose.[1]
Firearms
Nitrates are used as oxidizing agents, most notably in explosives, where the rapid oxidation of carbon compounds liberates large volumes of gases (see gunpowder for an example).
Industrial
Sodium nitrate is used to remove air bubbles from molten glass and some ceramics. Mixtures of the molten salt are used to harden some metals.[1]
Cinema
Nitrate was also used as a film stock through nitrocellulose. Due to its high combustibility, the studios swapped to acetate safety film in 1950.
Medicine
In the medical field, nitrates, such as glyceryl trinitrate, isosorbide dinitrate, and isosorbide mononitrate, are used in the prophylaxis and management of acute coronary syndrome, myocardial infarction, acute pulmonary oedema.[8] These class of drugs are also known as nitrovasodilators.
Detection
Almost all methods for detection of nitrate rely on its conversion to nitrite followed by nitrite-specific tests. The reduction of nitrate to nitrite is effected by copper-cadmium material. The sample is introduced with a flow injection analyzer, and the resulting nitrite-containing effluent is then combined with a reagent for colorimetric or electrochemical detection. The most popular of these assays is the Griess test, whereby nitrite is converted to a deeply colored azo dye suited for UV-vis spectroscopic analysis. The method exploits the reactivity of nitrous acid derived from acidification of nitrite. Nitrous acid selectively reacts with aromatic amines to give diazonium salts, which in turn couple with a second reagent to give the azo dye. The detection limit is 0.02 to 2 μM.[9] Such methods have been highly adapted to biological samples.[10]
Safety
The acute toxicity of nitrate is low. "Substantial disagreement" exists about the long-term risks of nitrate exposure. The two areas of possible concern are that (i) nitrate could be a precursor to nitrite in the lower gastrointestinal tract, and nitrite is a precursor to nitrosamines, which are implicated in carcinogenesis, and (ii) nitrate is implicated in methemoglobinemia, a disorder of hemoglobin in red blood cells.[11][12]
Methemoglobinemia
Nitrates do not affect infants and pregnant women.[13][14] Blue baby syndrome is caused by a number of other factors such as gastric upset, such as diarrheal infection, protein intolerance, heavy metal toxicity etc., with nitrates playing a minor role.[15]
Drinking water standards
Through the Safe Drinking Water Act, the United States Environmental Protection Agency has set a maximum contaminant level of 10 mg/L or 10 ppm of nitrate in drinking water.[16]
An acceptable daily intake (ADI) for nitrate ions was established in the range of 0–3.7 mg (kg body weight)−1 day−1 by the Joint FAO/WHO Expert Committee on Food Additives (JEFCA).[17]
Aquatic toxicity
In freshwater or estuarine systems close to land, nitrate can reach concentrations that are lethal to fish. While nitrate is much less toxic than ammonia,[18] levels over 30 ppm of nitrate can inhibit growth, impair the immune system and cause stress in some aquatic species.[19] Nitrate toxicity remains a subject of debate.[20]
In most cases of excess nitrate concentrations in aquatic systems, the primary sources are wastewater discharges, as well as surface runoff from agricultural or landscaped areas that have received excess nitrate fertilizer. The resulting eutrophication and algae blooms result in anoxia and dead zones. As a consequence, as nitrate forms a component of total dissolved solids, they are widely used as an indicator of water quality.
Domestic animal feed
Symptoms of nitrate poisoning in domestic animals include increased heart rate and respiration; in advanced cases blood and tissue may turn a blue or brown color. Feed can be tested for nitrate; treatment consists of supplementing or substituting existing supplies with lower nitrate material. Safe levels of nitrate for various types of livestock are as follows:[21]
| Category | %NO3 | %NO3–N | %KNO3 | Effects |
|---|---|---|---|---|
| 1 | < 0.5 | < 0.12 | < 0.81 | Generally safe for beef cattle and sheep |
| 2 | 0.5–1.0 | 0.12–0.23 | 0.81–1.63 | Caution: some subclinical symptoms may appear in pregnant horses, sheep and beef cattle |
| 3 | 1.0 | 0.23 | 1.63 | High nitrate problems: death losses and abortions can occur in beef cattle and sheep |
| 4 | < 1.23 | < 0.28 | < 2.00 | Maximum safe level for horses. Do not feed high nitrate forages to pregnant mares |
The values above are on a dry (moisture-free) basis.
Salts and covalent derivatives
Nitrate formation with elements of the periodic table:
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)−4 | C | NO−3, NH4NO3 |
O | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd(NO3)3 | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
See also
- Ammonium
- Eutrophication
- f-ratio
- Nitrification
- Nitratine
- Nitrite, the anion NO−2
- Nitrogen oxide
- Nitrogen trioxide, the neutral radical NO3
- Peroxynitrate, OONO–2
- Sodium nitrate
References
- ↑ 1.0 1.1 1.2 1.3 Laue, Wolfgang; Thiemann, Michael; Scheibler, Erich; Wiegand, Karl Wilhelm (2006). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a17_265.
- ↑ 2.0 2.1 "Food sources of nitrates and nitrites: the physiologic context for potential health benefits". The American Journal of Clinical Nutrition 90 (1): 1–10. July 2009. doi:10.3945/ajcn.2008.27131. PMID 19439460.
- ↑ "The Effect of Dietary Nitrate Supplementation on Endurance Exercise Performance in Healthy Adults: A Systematic Review and Meta-Analysis". Sports Medicine (Auckland, N.Z.) 47 (4): 735–756. April 2017. doi:10.1007/s40279-016-0617-7. PMID 27600147. http://espace.library.uq.edu.au/view/UQ:407817/uq407817_OA.pdf.
- ↑ Maughan, Ronald J (2013). Food, Nutrition and Sports Performance III. New York: Taylor & Francis. pp. 63. ISBN 978-0-415-62792-4.
- ↑ "Effect of white versus red meat on endogenous N-nitrosation in the human colon and further evidence of a dose response". The Journal of Nutrition 132 (11 Suppl): 3522S–3525S. November 2002. doi:10.1093/jn/132.11.3522S. PMID 12421881.
- ↑ "Sodium nitrite: the "cure" for nitric oxide insufficiency". Meat Science 92 (3): 274–9. November 2012. doi:10.1016/j.meatsci.2012.03.001. PMID 22464105.
- ↑ "Is There a Difference Between Cured and Uncured Bacon?". 9 December 2022. https://www.tastingtable.com/1132614/is-there-a-difference-between-cured-and-uncured-bacon/.
- ↑ Soman, Biji; Vijayaraghavan, Govindan. "The role of organic nitrates in the optimal medical management of angina". https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15/The-role-of-organic-nitrates-in-the-optimal-medical-management-of-angina,%20https://www.escardio.org/Journals/E-Journal-of-Cardiology-Practice/Volume-15/The-role-of-organic-nitrates-in-the-optimal-medical-management-of-angina.
- ↑ Moorcroft, M.; Davis, J.; Compton, R. G. (2001). "Detection and determination of nitrate and nitrite: A review". Talanta 54 (5): 785–803. doi:10.1016/S0039-9140(01)00323-X. PMID 18968301.
- ↑ Ellis, Graham; Adatia, Ian; Yazdanpanah, Mehrdad; Makela, Sinikka K. (1998). "Nitrite and Nitrate Analyses: A Clinical Biochemistry Perspective". Clinical Biochemistry 31 (4): 195–220. doi:10.1016/S0009-9120(98)00015-0. PMID 9646943.
- ↑ Powlson, David S.; Addiscott, Tom M.; Benjamin, Nigel; Cassman, Ken G.; De Kok, Theo M.; Van Grinsven, Hans; l'Hirondel, Jean-Louis; Avery, Alex A. et al. (2008). "When Does Nitrate Become a Risk for Humans?". Journal of Environmental Quality 37 (2): 291–5. doi:10.2134/jeq2007.0177. PMID 18268290. http://digitalcommons.unl.edu/cgi/viewcontent.cgi?article=1102&context=agronomyfacpub.
- ↑ "Nitrate and Nitrite Poisoning: Introduction". The Merck Veterinary Manual. http://www.merckvetmanual.com/mvm/index.jsp?cfile=htm/bc/212300.htm.
- ↑ Addiscott, T.M.; Benjamin, N. (2006). "Nitrate and human health". Soil Use and Management 20 (2): 98–104. doi:10.1111/j.1475-2743.2004.tb00344.x.
- ↑ A. A. Avery: Infant Methemoglobinemia - Reexamining the Role of Drinking Water Nitrates, Environmental Health Perspectives, Volume 107, Number 7, July 1999.
- ↑ "Nitrates in drinking water and methemoglobin levels in pregnancy: a longitudinal study" (in En). Environmental Health 9 (1): 60. October 2010. doi:10.1186/1476-069x-9-60. PMID 20946657.
- ↑ "4. What are EPA's drinking water regulations for nitrate?" (in en-US). https://safewater.zendesk.com/hc/en-us/articles/211401718-4-What-are-EPA-s-drinking-water-regulations-for-nitrate-.
- ↑ Bagheri, H.; Hajian, A.; Rezaei, M.; Shirzadmehr, A. (2017). "Composite of Cu metal nanoparticles-multiwall carbon nanotubes-reduced graphene oxide as a novel and high performance platform of the electrochemical sensor for simultaneous determination of nitrite and nitrate". Journal of Hazardous Materials 324 (Pt B): 762–772. doi:10.1016/j.jhazmat.2016.11.055. PMID 27894754.
- ↑ "Acute toxicity of sodium nitrate, potassium nitrate, and potassium chloride and their effects on the hemolymph composition and gill structure of early juvenile blue swimmer crabs(Portunus pelagicus Linnaeus, 1758) (Decapoda, Brachyura, Portunidae)". Environmental Toxicology and Chemistry 26 (9): 1955–62. September 2007. doi:10.1897/07-144r.1. PMID 17705664.
- ↑ Sharpe, Shirlie. "Nitrates in the Aquarium". About.com. http://freshaquarium.about.com/od/watercare/a/nitrates.htm.
- ↑ "Effects of potassium on nitrate mediated alterations of osmoregulation in marine crabs". Aquatic Toxicology 85 (3): 202–8. December 2007. doi:10.1016/j.aquatox.2007.09.004. PMID 17942166.
- ↑ "Nitrate Risk in Forage Crops - Frequently Asked Questions". Agriculture and Rural Development. Government of Alberta. http://www1.agric.gov.ab.ca/$department/deptdocs.nsf/all/faq8911.
External links
| HNO3 | He | ||||||||||||||||
| LiNO3 | Be(NO3)2 | B(NO3)−4 | C | NO−3, NH4NO3 |
O | FNO3 | Ne | ||||||||||
| NaNO3 | Mg(NO3)2 | Al(NO3)3 | Si | P | S | ClONO2 | Ar | ||||||||||
| KNO3 | Ca(NO3)2 | Sc(NO3)3 | Ti(NO3)4 | VO(NO3)3 | Cr(NO3)3 | Mn(NO3)2 | Fe(NO3)3, Fe(NO3)2 |
Co(NO3)2, Co(NO3)3 |
Ni(NO3)2 | Cu(NO3)2 | Zn(NO3)2 | Ga(NO3)3 | Ge | As | Se | Br | Kr |
| RbNO3 | Sr(NO3)2 | Y(NO3)3 | Zr(NO3)4 | Nb | Mo | Tc | Ru | Rh | Pd(NO3)2 | AgNO3 | Cd(NO3)2 | In | Sn | Sb(NO3)3 | Te | I | Xe(NO3)2 |
| CsNO3 | Ba(NO3)2 | Hf | Ta | W | Re | Os | Ir | Pt | Au | Hg2(NO3)2, Hg(NO3)2 |
Tl(NO3)3, TlNO3 |
Pb(NO3)2 | Bi(NO3)3 BiO(NO3) |
Po | At | Rn | |
| FrNO3 | Ra(NO3)2 | Rf | Db | Sg | Bh | Hs | Mt | Ds | Rg | Cn | Nh | Fl | Mc | Lv | Ts | Og | |
| ↓ | |||||||||||||||||
| La(NO3)3 | Ce(NO3)3, Ce(NO3)4 |
Pr | Nd(NO3)3 | Pm | Sm | Eu(NO3)3 | Gd(NO3)3 | Tb(NO3)3 | Dy | Ho | Er | Tm | Yb | Lu | |||
| Ac(NO3)3 | Th(NO3)4 | Pa | UO2(NO3)2 | Np | Pu | Am | Cm | Bk | Cf | Es | Fm | Md | No | Lr | |||
 |