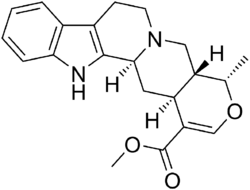
Chemistry:Ajmalicine
 | |
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H24N2O3 |
| Molar mass | 352.434 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 262.5 to 263 °C (504.5 to 505.4 °F) |
| |
| |
| | |
Ajmalicine, also known as δ-yohimbine or raubasine, is an antihypertensive drug used in the treatment of high blood pressure.[1] It has been marketed under numerous brand names including Card-Lamuran, Circolene, Cristanyl, Duxil, Duxor, Hydroxysarpon, Iskedyl, Isosarpan, Isquebral, Lamuran, Melanex, Raunatin, Saltucin Co, Salvalion, and Sarpan.[1] It is an alkaloid found naturally in various plants such as Rauvolfia spp., Catharanthus roseus, and Mitragyna speciosa.[1][2][3]
Ajmalicine is structurally related to yohimbine, rauwolscine, and other yohimban derivatives.[4] Like corynanthine, it acts as a α1-adrenergic receptor antagonist with preferential actions over α2-adrenergic receptors, underlying its hypotensive rather than hypertensive effects.[1][5]
Additionally, it is a very strong inhibitor of the CYP2D6 liver enzyme, which is responsible for the breakdown of many drugs. Its binding affinity at this receptor is 3.30 nM.[6]
Biosynthesis
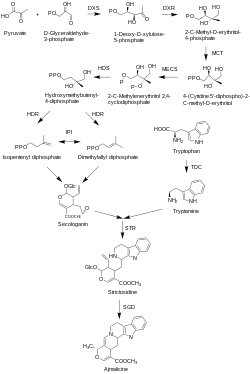
Two moieties are involved in the biosynthesis of ajmalicine, the terpenoid moiety and the indole moiety.[7] The terpenoid moiety is synthesized by the MEP pathway. The MEP pathway starts with pyruvate and D-glyceraldehyde-3-phosphate, followed by the involvement of DXS, DXR, MCT, MECS, HDS, and HDR genes. This results in isopentenyl diphosphate and dimethylallyl diphosphate which are then synthesized into secologanin. The indole moiety is brought about by the indole pathway, where tryptophan decarboxylase (TDC) catalyzes the formation of tryptamine from tryptophan. Strictosidine synthase (STR) then catalyzes the formation of strictosidine from the intermediates of the previous pathways. Strictosidine is the common precursor for all terpenoid indole alkaloids. Ajmalicine is finally synthesized under catalysis of strictosidine glucosidase (SGD).
See also
References
- ↑ 1.0 1.1 1.2 1.3 "Compartmentation of alkaloid synthesis, transport. and storage". Alkaloids: biochemistry, ecology, and medicinal applications. New York: Plenum Press. 1998. ISBN 0-306-45465-3. https://books.google.com/books?id=bMCzyrAtrvYC&q=ajmalicine&pg=PA451.
- ↑ "Alkaloid Production in Catharanthus roseus cell cultures VIII". Planta Medica 42 (1): 22–31. May 1981. doi:10.1055/s-2007-971541. PMID 17401876.
- ↑ "Phytochemical characterization of the leaves of Mitragyna speciosa grown in U.S.A". Natural Product Communications 4 (7): 907–910. July 2009. doi:10.1177/1934578X0900400705. PMID 19731590.
- ↑ (in en) Alkaloids: Biochemistry, Ecology, and Medicinal Applications. Springer Science & Business Media. 1998-06-30. ISBN 978-0-306-45465-3.
- ↑ "Inhibition of the alpha 1 and alpha 2-adrenoceptor-mediated pressor response in pithed rats by raubasine, tetrahydroalstonine and akuammigine". European Journal of Pharmacology 106 (1): 203–205. October 1984. doi:10.1016/0014-2999(84)90698-8. PMID 6099269.
- ↑ "Development of a pharmacophore for inhibition of human liver cytochrome P-450 2D6: molecular modeling and inhibition studies". Journal of Medicinal Chemistry 36 (9): 1136–1145. April 1993. doi:10.1021/jm00061a004. PMID 8487254.
- ↑ 7.0 7.1 "Abscisic Acid Enhanced Ajmalicine Biosynthesis in Hairy Roots of Rauvolfia verticillata by Upregulating Expression of the MEP Pathway Genes". Russian Journal of Plant Physiology 61 (1): 136–141. 2014. doi:10.1134/S102144371401004X. ISSN 1021-4437.
 |

