Biology:Hydroxyprogesterone caproate
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Delalutin, Proluton, Makena, others |
| Other names | OHPC; Hydroxyprogesterone capronate; Hydroxyprogesterone hexanoate; 17α-Hydroxyprogesterone caproate; 17α-OHPC; 17-Hydroxyprogesterone caproate; 17-OHPC; 17-HPC; 17α-HPC; HPC; LPCN-1107; 17α-Hydroxypregn-4-ene-3,20-dione 17α-hexanoate |
| Routes of administration | Intramuscular injection,[1] subcutaneous autoinjection[2][3] |
| Drug class | Progestogen; Progestin; Progestogen ester; Antigonadotropin |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | Oral: Very low (~3% in rats)[4] Intramuscular: 100% (in rats)[4] |
| Protein binding | Extensive (to albumin, not to CBG or (likely) SHBG)[1][5][6] |
| Metabolism | Reduction and hydroxylation (via CYP3A4, CYP3A5, CYP3A7) and conjugation (glucuronidation, sulfation, acetylation)[1] |
| Elimination half-life | Non-pregnant: 7.8 days[7][8] Singlet: 16–17 days[1][9] Twins: 10 days[9] |
| Excretion | Feces: 50%[1] Urine: 30%[1] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C27H40O4 |
| Molar mass | 428.613 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Hydroxyprogesterone caproate, sold under the brand names Proluton and Makena among others, is a medication used to reduce the risk of preterm birth in women pregnant with one baby who have a history of spontaneous preterm birth.[10] In March 2023, the manufacturer, Covis Pharma, agreed to withdraw the drug from the US market.[11][12][13] The approvals of Makena and its generics were withdrawn by the US Food and Drug Administration (FDA) in April 2023.[10]
Hydroxyprogesterone caproate is a progestin medication which was used to prevent preterm birth in pregnant women with a history of the condition and to treat gynecological disorders.[1][8][9][14][3] It has also been formulated in combination with estrogens for various indications (brand names Gravibinon and Primosiston) and as a form of long-lasting injectable birth control (brand name Chinese Injectable No. 1).[15] It is not used by mouth and is instead given by injection into muscle or fat, typically once per week to once per month depending on the indication.[1][4][3]
Hydroxyprogesterone caproate is generally well tolerated and produces few side effects.[1] Injection site reactions such as pain and swelling are the most common side effect of hydroxyprogesterone caproate.[1] The medication may increase the risk of gestational diabetes when used in pregnant women.[1][16] Hydroxyprogesterone caproate is a progestin, or a synthetic progestogen, and hence is an agonist of the progesterone receptor, the biological target of progestogens like progesterone.[16] It has some antimineralocorticoid activity and no other important hormonal activity.[17][18][19][20][21] The medication shows a number of differences from natural progesterone.[16][22]
Hydroxyprogesterone caproate was discovered in 1953 and was introduced for medical use in 1954 or 1955.[23] It was marketed in the United States under the brand name Delalutin and throughout Europe under the brand name Proluton.[24] The medication was discontinued in the United States in 1999.[25] However, hydroxyprogesterone caproate was subsequently reintroduced in the United States under the brand name Makena for the treatment of preterm birth in 2011.[26]
Medical uses
Preterm birth
The use of hydroxyprogesterone caproate in pregnancy to prevent preterm birth in women with a history of preterm delivery between 20 weeks and 36 weeks and 6 days is supported by the Society of Maternal Fetal Medicine Clinic Guidelines put out in May 2012 as Level I and III evidence, Level A recommendation.[27] Level I evidence refers to a properly powered randomized controlled trial, and level III evidence is support from expert opinion, while a Level A recommendation confers that the recommendation is made based on good and consistent scientific evidence. Hydroxyprogesterone caproate 250 mg IM weekly preferably starting at 16–20 weeks until 36 weeks is recommended. In these women, if the transvaginal ultrasound cervical length shortens to <25 mm at < 24 weeks, cervical cerclage may be offered. In the 2013 study the guideline recommendation is based on,[28] there was also a significant decrease of neonatal morbidity including lower rates of necrotizing enterocolitis (0 in the treatment group vs 4 in the control), intraventricular hemorrhage (4 in the treatment group compared with 8 in the control for a relative risk of 0.25), and need for supplemental oxygen (14% in the treatment group vs 24% in the placebo for a relative risk of 0.42). Furthermore, this study contained 463 women, 310 of whom received injection. Of these women, 9 had infants with congenital malformations (2%), but there was no consistent pattern and none involved internal organs.
There is no evidence of fetal risk with use of hydroxyprogesterone caproate during pregnancy. A review concluded that information about the potential harms was lacking.[29] Three clinical studies in singleton pregnancies of 250 mg/week of intramuscular hydroxyprogesterone caproate have all shown a trend for an increase in pregnancy loss due to miscarriage compared to placebo.[30][31][28] One of them, a large National Institutes of Health (NIH) study in 2003, looked at the effect of hydroxyprogesterone caproate injections in women at risk for repeat premature birth and found that the treated group experienced premature birth in 37% versus 55% in the controls.[28] A follow-up study of the offspring showed no evidence that hydroxyprogesterone caproate affected the children in the first years of life.[32] Based on these NIH data, hydroxyprogesterone caproate was approved by the US Food and Drug Administration (FDA) in 2011, as a medication to reduce the risk of premature birth in selected women at risk.[citation needed]
The FDA expressed concern about miscarriage at the 2006 advisory committee meeting; the committee voted unanimously that further study was needed to evaluate the potential association of hydroxyprogesterone caproate with increased risk of second trimester miscarriage and stillbirth.[33] A toxicology study in rhesus monkeys resulted in the death of all rhesus fetuses exposed to 1 and 10 times the human dose equivalent of hydroxyprogesterone caproate.[34] As of 2008[update], hydroxyprogesterone caproate was a category D progestin according to the FDA (that is, there is evidence of fetal harm). There is speculation that the castor oil in the hydroxyprogesterone caproate formulation may not be beneficial for pregnancy.[35][36] Of note, the above-mentioned NEJM study by Meirs et al. compares the effect of hydroxyprogesterone caproate (with the castor oil component) to castor oil injection as the placebo.
A study published in February 2016, found amongst other findings:[37]
OPPTIMUM strongly suggests that the efficacy of progesterone in improving outcomes is either non-existent or weak. Given the heterogeneity of the preterm labour syndrome we cannot exclude benefit in specific phenotypic or genotypic subgroups of women at risk. However, the subgroups of women who might benefit do not appear to be easily identifiable by current selection strategies, including cervical length measurement and fibronectin testing.
Reassuringly, our study suggests that progesterone is safe for those who wish to take it for preterm birth prophylaxis. The overall rate of maternal or child adverse events was similar in the progesterone and placebo groups. There were few differences in the incidence of adverse secondary outcomes in the two groups, with the exception of a higher rate of renal, gastrointestinal, and respiratory complications in childhood in the progesterone groups. Importantly, the absolute rates of these complications was low. Follow-up of other babies exposed in utero to vaginal progesterone would be helpful in determining whether the increased rate of some renal, gastrointestinal, and respiratory complications is a real effect or a type I error.
The journal reviewer made the following notable commentary on the OPPTIMUM study: "That's it. This story is ended, and nobody need ever use vaginal progesterone again to prevent preterm birth."[38]
A Cochrane review on progestogen for preventing preterm birth concluded that there was little evidence that either vaginal or intramuscular progesterone helped to reduce the risk of preterm birth in women with a multiple pregnancy.[39]
Gynecological disorders
Hydroxyprogesterone caproate is used in the treatment of threatened miscarriage, gynecological disorders such as dysmenorrhea, premenstrual syndrome, fibrocystic breast disease, adenosis, and breast pain.[9] In addition, hydroxyprogesterone caproate is used in the treatment of endometrial cancer and has been found to be significantly effective in extending life in both premenopausal and postmenopausal women with the disease.[40] The medication was used widely in the 1950s through the 1970s for such indications, but hydroxyprogesterone caproate more recently has received the most attention in the prevention of preterm birth.[9]
Birth control
Hydroxyprogesterone caproate is available in combination with estradiol valerate as a once-monthly combined injectable contraceptive in a some countries.[15][41]
Other uses
Hydroxyprogesterone caproate has been used as a component of menopausal hormone therapy in women.[42][43]
Hydroxyprogesterone caproate has been used to treat benign prostatic hyperplasia in men, although evidence of effectiveness is marginal and uncertain.[44] It has also been used to treat prostate cancer, at a dosage of 1,500 mg twice per week.[45][46][47][48] The mechanism of action of hydroxyprogesterone caproate in these uses is suppression of testicular androgen production via suppression of luteinizing hormone secretion, which are the result of the progestogenic and antigonadotropic activity of hydroxyprogesterone caproate.[44] However, symptoms of hypogonadism may develop when hydroxyprogesterone caproate is used for this indication, with two-thirds of men reportedly experiencing impotence.[49]
Hydroxyprogesterone caproate has been used as a component of feminizing hormone therapy for transgender women.[50][51][52][53][54] Due to micronization, bioidentical progestogens are more commonly used.
Available forms
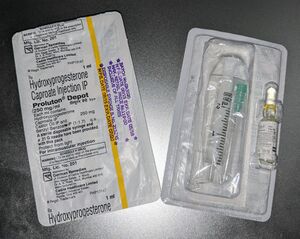
Hydroxyprogesterone caproate is available alone in the form of ampoules and vials of 125 and 250 mg/mL oil solutions for intramuscular injection (brand names Proluton, Makena).[55][56] It is also available alone in the form of a 250 mg/mL autoinjector for use by subcutaneous injection (brand name Makena).[3]
Hydroxyprogesterone caproate is or was available in combination with estradiol valerate in the form of ampoules and vials of 250 mg/mL OHPC and 5 mg/mL estradiol valerate oil solutions for intramuscular injection (brand names Gravibinon, Chinese Injectable No. 1).[57][58][59][60] The medication is or was available in combination with estradiol benzoate in the form of ampoules of 125–250 mg OHPC and 10 mg estradiol benzoate in oil solution for intramuscular injection (brand name Primosiston) as well.[61][62][63][64][65]: 1045 In addition, hydroxyprogesterone caproate has been marketed in combination with estradiol dipropionate in the form of 50 mg/mL hydroxyprogesterone caproate and 1 mg/mL estradiol dipropionate (brand name EP Hormone Depot) in Japan.[66][67]
Contraindications
Contraindications of hydroxyprogesterone caproate include previous or current thrombosis or thromboembolic disease, known or suspected breast cancer, past or present history of other hormone-sensitive cancer, undiagnosed abnormal vaginal bleeding unrelated to pregnancy, cholestatic jaundice of pregnancy, liver tumors or active liver disease, and uncontrolled hypertension.[3] A few relative contraindications also exist for hydroxyprogesterone caproate.[3]
Side effects
Hydroxyprogesterone caproate is generally well tolerated and produces relatively few side effects.[1] Injection site reactions such as pain, soreness, swelling, itching, bruising, and lumps are the most common side effect of hydroxyprogesterone caproate.[1] In contrast to large doses of progesterone however, which produce moderate-to-severe such reactions, hydroxyprogesterone caproate is relatively free from injection site reactions.[68] Side effects of hydroxyprogesterone caproate that occur in greater than or equal to 2% of users include injection site pain (34.8%), injection site swelling (17.1%), urticaria (12.3%), pruritus (7.7%), injection site pruritus (5.8%), nausea (5.8%), injection site nodules (4.5%), and diarrhea (2.3%).[3] Numerically increased rates relative to controls of miscarriage (2.4% vs. 0%), stillbirth (2.0% vs. 1.3%), admission for preterm labor (16.0% vs. 13.8%), preeclampsia or gestational hypertension (8.8% vs. 4.6%), gestational diabetes (5.6% vs. 4.6%),[1][16] and oligohydramnios (3.6% vs. 1.3%) have been observed with hydroxyprogesterone caproate in clinical trials in which it was given to pregnant women to prevent preterm birth.[3]
Overdose
There have been no reports of overdose of hydroxyprogesterone caproate.[3] In the event of overdose, treatment should be based on symptoms.[3] Hydroxyprogesterone caproate has been studied in humans at high doses of 2,000 to 5,000 mg per week by intramuscular injection, without safety concerns.[7][21][69][70]
Interactions
Hydroxyprogesterone caproate is not likely to affect most cytochrome P450 enzymes at therapeutic concentrations.[3] Drug interaction studies have not been performed with hydroxyprogesterone caproate.[3]
Pharmacology
Pharmacodynamics
Hydroxyprogesterone caproate has progestogenic activity, some antimineralocorticoid activity, and no other important hormonal activity.[17][8][18][19][69]
| Compound | hPR-A | hPR-B | rbPR | rbGR | rbER | |||
|---|---|---|---|---|---|---|---|---|
| Progesterone | 100 | 100 | 100 | <1 | <1 | |||
| 17α-Hydroxyprogesterone | 1 | 1 | 3 | 1 | <1 | |||
| Hydroxyprogesterone caproate | 26 | 30 | 28 | 4 | <1 | |||
| Hydroxyprogesterone acetate | 38 | 46 | 115 | 3 | ? | |||
| Notes: Values are percentages (%). Reference ligands (100%) were progesterone for the PR, dexamethasone for the GR, and estradiol for the ER. Sources: See template. | ||||||||
Progestogenic activity
Hydroxyprogesterone caproate, also known as 17α-hydroxyprogesterone caproate, is closer to progesterone in terms of structure and pharmacology than most other progestins, and is essentially a pure progestogen – that is, a selective agonist of the progesterone receptor (PR) with minimal or no other hormonal activity.[20][21] However, hydroxyprogesterone caproate has improved pharmacokinetics compared to progesterone, namely a much longer duration with intramuscular injection in oil solution.[9][71][62][72]
Administered by intramuscular injection, the endometrial transformation dosage of hydroxyprogesterone caproate per cycle is 250 to 500 mg, and the weekly substitution dosage of hydroxyprogesterone caproate is 250 mg, while the effective dosage of hydroxyprogesterone caproate in the menstrual delay test (Greenblatt) is 25 mg per week.[62][72][73] An effective ovulation-inhibiting dosage of hydroxyprogesterone caproate is 500 mg once per month by intramuscular injection.[59][74][75] However, the dose of hydroxyprogesterone caproate used in once-a-month combined injectable contraceptives is 250 mg, and this combination is effective for inhibition of ovulation similarly.[59][75] For comparison, the dose of medroxyprogesterone acetate (MPA; 6α-methyl-17α-hydroxyprogesterone acetate), a close analogue of hydroxyprogesterone caproate, used by intramuscular injection in microcrystalline aqueous suspension in once-a-month combined injectable contraceptives, is 25 mg.[59][74] It has also been said that given by intramuscular injection, 250 mg hydroxyprogesterone caproate in oil solution is equivalent in progestogenic potency to 50 mg medroxyprogesterone acetate in microcrystalline aqueous suspension.[76] Although the elimination half-life of intramuscular hydroxyprogesterone caproate in oil solution in non-pregnant women is about 8 days,[7][8] the elimination half-life of intramuscular medroxyprogesterone acetate in microcrystalline aqueous suspension in women is around 50 days.[77] Hydroxyprogesterone caproate is also to some degree less potent than the more closely related ester hydroxyprogesterone acetate (OHPA; 17α-hydroxyprogesterone acetate).[78]
17α-Hydroxyprogesterone (OHP) has weak progestogenic activity, but C17α esterification results in higher progestogenic activity.[65] Of a variety of different esters, the caproate (hexanoate) ester was found to have the strongest progestogenic activity, and this served as the basis for the development of hydroxyprogesterone caproate, as well as other caproate progestogen esters such as gestonorone caproate.[65] Hydroxyprogesterone caproate is a much more potent progestogen than 17α-hydroxyprogesterone, but does not have as high of affinity for the PR as progesterone.[78] Hydroxyprogesterone caproate has about 26% and 30% of the affinity of progesterone for the human PR-A and PR-B, respectively.[1][78] The medication was no more efficacious than progesterone in activating these receptors and eliciting associated gene expression in vitro.[1][78]
Antigonadotropic effects
Due to activation of the PR, hydroxyprogesterone caproate has antigonadotropic effects, or produces suppression of the hypothalamic–pituitary–gonadal axis,[79][80] and can significantly suppress gonadotropin secretion and gonadal sex hormone production at sufficiently high doses.[48] One study found that hydroxyprogesterone caproate by intramuscular injection at a dosage of 200 mg twice weekly for the first two weeks and then 200 mg once weekly for 12 weeks did not significantly influence urinary excretion of estrogens, luteinizing hormone, or follicle-stimulating hormone in men with benign prostatic hyperplasia.[81] In another study that used an unspecified dosage of intramuscular hydroxyprogesterone caproate, testosterone secretion was assessed in a single man and was found to decrease from 4.2 mg/day to 2.0 mg/day (or by approximately 52%) by 6 weeks of treatment, whereas secretion of luteinizing hormone remained unchanged in the man.[20] Yet another study found that 3,000 mg/week hydroxyprogesterone caproate by intramuscular injection suppressed testosterone levels from 640 ng/dL to 320–370 ng/dL (by 42–50%) in a single man with prostate cancer, which was similar to the testosterone suppression with cyproterone acetate or chlormadinone acetate.[82] Gestonorone caproate, a closely related progestin to hydroxyprogesterone caproate with about 5- to 10-fold greater potency in humans,[83][84] was found to suppress testosterone levels by 75% at a dosage of 400 mg/week in men with prostate cancer.[85][86] For comparison, orchiectomy decreased testosterone levels by 91%.[85] In general, progestins are able to maximally suppress testosterone levels by about 70 to 80%.[87][88][89][85][86] The antigonadotropic effects of hydroxyprogesterone caproate and hence its testosterone suppression are the basis of the use of hydroxyprogesterone caproate in the treatment of benign prostatic hyperplasia and prostate cancer in men.[44][45][47][48] Suppression of luteinizing hormone levels by hydroxyprogesterone caproate has also been observed in women.[90][84]
Glucocorticoid activity
Hydroxyprogesterone caproate is said not to have any glucocorticoid activity.[21] In accordance, hydroxyprogesterone caproate has been found not to alter cortisol levels in humans even with very high doses by intramuscular injection.[7] This is of relevance because medications with significant glucocorticoid activity suppress cortisol levels due to increased negative feedback on the hypothalamic–pituitary–adrenal axis.[55][91][92] Hydroxyprogesterone caproate has been studied in humans at doses as high as 5,000 mg per week by intramuscular injection, with safety and without glucocorticoid effects observed.[7][70] The medication does interact with the glucocorticoid receptor however; it has about 4% of the affinity of dexamethasone for the rabbit glucocorticoid receptor.[1][78] But it acts as a partial agonist of the receptor and has no greater efficacy than progesterone in activating the receptor and eliciting associated gene expression in vitro.[1][78][93]
Other activities
As a pure progestogen, hydroxyprogesterone caproate has no androgenic, antiandrogenic, estrogenic, or glucocorticoid activity.[20][21][94] The absence of androgenic and antiandrogenic activity with hydroxyprogesterone caproate is in contrast to most other 17α-hydroxyprogesterone-derivative progestins.[71][94] Due to its lack of androgenic properties, similarly to progesterone, hydroxyprogesterone caproate does not have any teratogenic effects on the fetus, making it safe for use during pregnancy.[21] Although hydroxyprogesterone caproate has been described as a pure progestogen, there is evidence that it possesses some antimineralocorticoid activity, similarly to progesterone and 17α-hydroxyprogesterone.[18][95][19] This includes clinically important diuretic effects and reversal of estrogen-induced fluid retention and edema.[95] Unlike progesterone, hydroxyprogesterone caproate and its metabolites are not anticipated to interact with non-genomic receptors such as membrane progesterone receptors or the GABAA receptor.[22] In accordance, hydroxyprogesterone caproate is not thought to possess the neurosteroid activities of progesterone or its associated sedative effects.[22]
In relation to cytochrome P450 enzymes, hydroxyprogesterone caproate has no effect on CYP1A, CYP2D6, CYP2C9, or CYP3A4, but is a modest inducer of CYP2C19.[9]
Differences from progesterone
There are pharmacodynamic differences between progesterone and hydroxyprogesterone caproate, which may have implications for obstetrical use.[16][22] These include:[16][22]
- Decreased myometrial activity with progesterone in vitro but no effect or increased myometrial activity with hydroxyprogesterone caproate[96]
- Prevention of cervical ripening with progesterone but unknown effect with hydroxyprogesterone caproate
- A non-significantly increased rate of stillbirth and miscarriages with hydroxyprogesterone caproate (in one study)
- A possibly increased incidence of gestational diabetes with hydroxyprogesterone caproate (increased in two studies, no difference in one study) but no such effect with progesterone
- A significantly increased risk of perinatal adverse effects such as fetal loss and preterm delivery in multiple gestations with hydroxyprogesterone caproate (in two studies)
Differences in the metabolism of progesterone and hydroxyprogesterone caproate and differences in the formation and activities of metabolites may be responsible for or involved in these observed biological and pharmacological differences.[22] Progesterone is metabolized by 5α- and 5β-reductases, 3α- and 3β-hydroxysteroid dehydrogenases, and 20α- and 20β-hydroxysteroid dehydrogenase in various tissues.[22][97] In target tissues, particularly the cervix and myometrium, these enzymes regulate local progesterone concentrations and can activate or inactivate progesterone signaling.[22] In addition, these enzymes catalyze the formation of metabolites of progesterone such as 5β-dihydroprogesterone and allopregnanolone, which signal through their own non-genomic receptors such as membrane progesterone receptors and the GABAA receptor and have their own important effects in pregnancy.[96][98][99] As examples, 5β-dihydroprogesterone has been found to play an important role in suppressing myometrial activity while allopregnanolone has potent sedative and anesthetic effects in the mother and especially the fetus and is involved in fetal nervous system development.[22][98][99][100][101] In contrast to progesterone, hydroxyprogesterone caproate is not metabolized by traditional steroid-transforming enzymes and instead is metabolized exclusively via oxidation at the caproate side chain by cytochrome P450 enzymes.[22] As such, it is not thought to have the same tissue-specific activation and inactivation patterns that progesterone does nor the same non-genomic actions that progesterone and its metabolites possess.[22]
Further clinical research is anticipated to provide additional data to help clarify the issue of safety with hydroxyprogesterone caproate.[16] In any case, it has been recommended by the American College of Obstetricians and Gynecologists that pregnant women treated with hydroxyprogesterone caproate receive counseling about its risks and benefits.[16]
Pharmacokinetics
| Parameter | Singleton | Twin |
|---|---|---|
| Cmax (ng/mL) | 22.6 (15.8–27.4) | 17.3 (12–27) |
| Cmean(0–t) (ng/mL) | 16.8 (12.8–22.7) | 12.3 (8.4–18.7) |
| Ctrough (ng/mL) | 14.1 (10–18.1) | 11.2 (4.8–16.3) |
| AUC0–t (ng/mL/day) | 117.3 (89.9–159.1) | 86.1 (59–131) |
| t1/2 (days) | 16.2 (10.6–21.0) | 10 (6–16) |
| Tmax (days) | 1.0 (1–3) | 1.2 (1–2) |
| Vd/F (×103) (L) | 56 (25.2–69.6) | 16.9 (9.1–24.5) |
| Cl/F (×103) (L) | 2.1 (1.5–2.7) | 1.2 (0.9–1.7) |
| Footnotes: a = OHPC 250 mg once per week by intramuscular injection. Sources: [9][102][103] | ||
Absorption
In animals, the bioavailability of hydroxyprogesterone caproate with intramuscular injection is nearly 100%, but its oral bioavailability is very low at less than 3%.[4] In women, 70 mg/day oral hydroxyprogesterone caproate has similar endometrial potency as 70 mg/day oral OHPA and 2.5 mg/day oral medroxyprogesterone acetate, indicating that oral hydroxyprogesterone caproate and OHPA have almost 30-fold lower potency than medroxyprogesterone acetate via oral administration.[104] Studies on progestogenic endometrial changes with oral hydroxyprogesterone caproate in women are mixed however, with one finding weak effects with 100 mg/day whereas another found that doses of 250 to 1,000 mg produced no effects.[105][106] As a result of its low oral potency, hydroxyprogesterone caproate has not been used by the oral route and has instead been administered by intramuscular injection.[4] However, a novel oral formulation of hydroxyprogesterone caproate (developmental code name LPCN-1107) is under development and has been found to be effective, though it required administration twice a day in a clinical study.[107][108][109]
A depot effect occurs when hydroxyprogesterone caproate is injected intramuscularly or subcutaneously, such that the medication has a prolonged duration of action.[2][9] Following a single intramuscular injection of 1,000 mg hydroxyprogesterone caproate in five women with endometrial cancer, peak levels of hydroxyprogesterone caproate were 27.8 ± 5.3 ng/mL and the time to peak concentrations was 4.6 ± 1.7 (3–7) days.[7][110] Following 13 weeks of continuous administration of 1,000 mg hydroxyprogesterone caproate per week, trough levels of hydroxyprogesterone caproate were 60.0 ± 14 ng/mL.[7][110] The pharmacokinetic parameters of 250 mg hydroxyprogesterone caproate once per week by intramuscular injection have also been studied in pregnant women with singleton and multiple (twin and triplet) gestation.[9][102][103] Steady state levels of the medication are achieved within 4 to 12 weeks of administration in pregnant women.[1] The duration of clinical biological effect of hydroxyprogesterone caproate by intramuscular injection has also been studied in women.[111] A single intramuscular injection of 65 to 500 mg hydroxyprogesterone caproate in oil solution has been found to have a duration of action of 5 to 21 days in terms of effect in the uterus and on body temperature in women.[111]
Hydroxyprogesterone caproate has been found to possess similar pharmacokinetics, including peak levels, time to peak levels, area-under-the-curve levels (i.e., total exposure), and elimination half-life, with administration via intramuscular injection or subcutaneous autoinjection.[2] However, there was a higher incidence of injection site pain with subcutaneous autoinjection than with intramuscular injection (37.3% vs. 8.2%).[2]
Distribution
Hydroxyprogesterone caproate is extensively bound to plasma proteins, of which include albumin.[1] Unlike progesterone and 17α-hydroxyprogesterone, hydroxyprogesterone caproate has very low affinity for corticosteroid-binding globulin (less than 0.01% of that of cortisol).[5] Progesterone and 17α-hydroxyprogesterone have low affinity for sex hormone-binding globulin, and for this reason, only a very small fraction of them (less than 0.5%) is bound to this protein in the circulation.[6]
Metabolism
Hydroxyprogesterone caproate appears to be metabolized primarily by the cytochrome P450 enzymes CYP3A4 and CYP3A5.[1] It may also be metabolized by CYP3A7 in fetal liver and the placenta.[1] Unlike progesterone, hydroxyprogesterone caproate is not metabolized by traditional steroid-transforming enzymes and does not form similar metabolites.[22] The metabolism of hydroxyprogesterone caproate is by reduction, hydroxylation, and conjugation, including glucuronidation, sulfation, and acetylation.[22] The caproate ester of hydroxyprogesterone caproate is not cleaved during metabolism, so 17α-hydroxyprogesterone is not formed from hydroxyprogesterone caproate.[22][78] As such, hydroxyprogesterone caproate is not a prodrug of 17α-hydroxyprogesterone, nor of progesterone.[22][78]
Hydroxyprogesterone caproate has been found to have an elimination half-life of 7.8 days when given by intramuscular injection in an oil-based formulation to non-pregnant women.[7][8] Its total duration is said to be 10 to 14 days, which is much longer than the duration of intramuscularly administered progesterone in an oil formulation (2 to 3 days).[112] In pregnant women, the elimination half-life of hydroxyprogesterone caproate appears to be longer, about 16 or 17 days.[1][9] However, in women pregnant with twins rather than a singlet, the elimination half-life of hydroxyprogesterone caproate was found to be shorter than this, at 10 days.[9] Hydroxyprogesterone caproate has been detected in pregnant women up to 44 days after the last dose.[9]
Elimination
Hydroxyprogesterone caproate is eliminated 50% in feces and 30% in urine when given by intramuscular injection to pregnant women.[1] Both the free steroid and conjugates are excreted by these routes, with the conjugates more prominent in feces.[1]
Time–concentration curves
Chemistry
Hydroxyprogesterone caproate, also known as 17α-hydroxyprogesterone caproate or as 17α-hydroxypregn-4-ene-3,20-dione 17α-hexanoate, is a synthetic pregnane steroid and a derivative of progesterone.[24][113] It is specifically a derivative of 17α-hydroxyprogesterone with a hexanoate (caproate) ester at the C17α position.[24][113] Analogues of hydroxyprogesterone caproate include other 17α-hydroxyprogesterone derivatives such as algestone acetophenide (dihydroxyprogesterone acetophenide), chlormadinone acetate, cyproterone acetate, hydroxyprogesterone acetate, hydroxyprogesterone heptanoate, medroxyprogesterone acetate, and megestrol acetate, as well as the caproate esters chlormadinone caproate, gestonorone caproate (norhydroxyprogesterone caproate), medroxyprogesterone caproate, megestrol caproate, and methenmadinone caproate.[24][113]
Synthesis
Chemical syntheses of hydroxyprogesterone caproate have been described.[114][115][116]: 6
History
Along with hydroxyprogesterone acetate, hydroxyprogesterone caproate was developed by Karl Junkmann of Schering AG in 1953 and was first reported by him in the medical literature in 1954.[117][118][119][120][121] It was reportedly first marketed in Japan in 1954 or 1955,[23] and was subsequently introduced as Delalutin in the United States in 1956.[9][122] Due to its much longer duration than parenteral progesterone, hydroxyprogesterone caproate had largely replaced progesterone in clinical practice by 1975.[123] After decades of use, Squibb, the manufacturer, voluntarily withdrew the Delalutin product in the United States in 1999.[25] Renewed interest in hydroxyprogesterone caproate in the United States was sparked with a large NIH-sponsored study in 2003 that found that hydroxyprogesterone caproate reduced the risk of premature birth in selected at-risk pregnant women.[28] With follow-up data showing no evidence of harmful effects on the offspring, the FDA approved the medication Makena, sponsored by KV Pharmaceutical, as an orphan drug in February 2011 to reduce the risk of premature birth in women prior to 37 weeks gestation with a single fetus who had at least one previous premature birth.[26][124]
Under the FDA Accelerated Approval Programs, drugs that fill an unmet need for serious conditions can be approved based on a surrogate endpoint. The pharmaceutical company is required to conduct confirmatory studies to show the drug provides a clinical benefit.[125] The confirmatory trial, the PROLONG study, was completed in 2019 and showed no benefit in preventing preterm birth.[126] The FDA proposed withdrawal of approval for Makena in 2020. [127]
Society and culture
Names
Hydroxyprogesterone caproate is the generic name of OHPC and its INN, USAN, BANM, and JAN, while hydroxyprogesterone hexanoate was its former BANM.[24][41][113]
Hydroxyprogesterone caproate is often mislabeled as and confused with progesterone and 17α-hydroxyprogesterone.[128] It should also not be confused with hydroxyprogesterone acetate, hydroxyprogesterone heptanoate, or medroxyprogesterone acetate.[113]
Hydroxyprogesterone caproate is marketed throughout the world under a variety of brand names including Proluton, Proluton Depot, and Makena (US), among many others.[24][41][113] It was also formerly marketed under brand names including Delalutin, Prodrox, and Hylutin among others, but these formulations have since been discontinued.[24][113] It has been marketed under the brand names Gravibinon and Injectable No. 1 (or Chinese Injectable No. 1) in combination with estradiol valerate[57][58][59][60] and under the brand name Primosiston in combination with estradiol benzoate.[61][62][63][64][65]
Availability
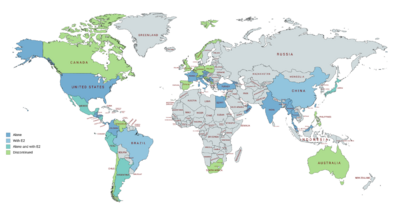
Hydroxyprogesterone caproate is marketed in the United States and throughout Europe, Asia, and Central and South America.[24][41][129] It is not available in Canada, the United Kingdom, New Zealand, or South Africa, and only veterinary formulations are available in Australia.[24][41][129] Hydroxyprogesterone caproate is also marketed in combination with estradiol valerate as a combined injectable contraceptive in a number of countries including in South America, Mexico, Japan, and China.[24][41][129] It has been marketed as an injectable preparation in combination with estradiol benzoate in some countries as well.[61][62][63][64][65]
Economics
With the designation of hydroxyprogesterone caproate as an orphan drug by the FDA and approval of Makena in 2011, the price of hydroxyprogesterone caproate in the United States was going to increase from US$15 to US$1,500 for a single dose, or from about US$300 to between US$25,000 and US$30,000 for a typical single month of treatment.[26] This was about a 100-fold increase in cost, with "minimal added clinical benefit", and was a strongly criticized pricing strategy.[26] The FDA subsequently announced that compounding pharmacies could continue to sell hydroxyprogesterone caproate at their usual cost of approximately US$10 to US$20 per dose without fear of legal reprisals.[26][130] KV Pharmaceutical also opted to reduced its price of Makena to US$690 per dose.[26][131] Hydroxyprogesterone caproate continued to be available at low cost from compounding pharmacies until late 2016, after which time the FDA published new guidance documents prohibiting compounding pharmacies from selling products that are "essentially copies" of commercially available drug products.[132][133]
Research
Cyclical therapy with 150 mg hydroxyprogesterone caproate by intramuscular injection was found to be effective in the treatment of 76 women with persistent, treatment-refractory acne in a preliminary study, with 84% responding to the therapy and experiencing a "good-to-excellent" improvement in symptoms.[112][134]
Hydroxyprogesterone caproate was studied by Schering for use as a progestogen-only injectable contraceptive at a dose of 250 to 500 mg once a month by intramuscular injection but produced poor cycle control at these doses and was never marketed.[135][136]
Hydroxyprogesterone caproate by itself has been found to have little or no effectiveness in the treatment of breast cancer in women.[65][137][138][139] Conversely, the combination of estradiol valerate and hydroxyprogesterone caproate has been found to be effective in the treatment of breast cancer in women.[65][95][140] Initial research based on limited clinical data reported that the breast-cancer response rate with a combination of estradiol valerate and hydroxyprogesterone caproate seemed to be greater than with an estrogen alone (35% vs. 50%).[65] However, subsequent research using the related but more potent progestin gestonorone caproate found that the combination of estradiol valerate and gestonorone caproate had effectiveness that was not significantly different from that of an estrogen alone in the treatment of breast cancer in women.[141]
A novel oral formulation of hydroxyprogesterone caproate (developmental code name LPCN-1107) is under development for the prevention of preterm labor.[107][108] As of September 2017, it is in phase II or phase III clinical trials for this indication.[107]
Veterinary uses
The pharmacokinetics of hydroxyprogesterone caproate in various ungulates including cattle, buffalo, sheep, and goat have been studied.[142]
References
- ↑ 1.00 1.01 1.02 1.03 1.04 1.05 1.06 1.07 1.08 1.09 1.10 1.11 1.12 1.13 1.14 1.15 1.16 1.17 1.18 1.19 1.20 1.21 1.22 1.23 1.24 "17 α-Hydroxyprogesterone caproate (Makena): in the prevention of preterm birth". Paediatric Drugs 13 (5): 337–45. October 2011. doi:10.2165/11208140-000000000-00000. PMID 21888448.
- ↑ 2.0 2.1 2.2 2.3 "Comparative Bioavailability of Hydroxyprogesterone Caproate Administered via Intramuscular Injection or Subcutaneous Autoinjector in Healthy Postmenopausal Women: A Randomized, Parallel Group, Open-label Study". Clinical Therapeutics 39 (12): 2345–2354. December 2017. doi:10.1016/j.clinthera.2017.10.020. PMID 29191450.
- ↑ 3.00 3.01 3.02 3.03 3.04 3.05 3.06 3.07 3.08 3.09 3.10 3.11 3.12 "Makena- hydroxyprogesterone caproate injection". 19 December 2022. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=a1998c1d-8337-4f00-8dcb-af3b54d39b77.
- ↑ 4.0 4.1 4.2 4.3 4.4 "Route of administration and formulation dependent pharmacokinetics of 17-hydroxyprogesterone caproate in rats". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 46 (2): 169–74. 2015. doi:10.3109/00498254.2015.1057547. PMID 26153441.
- ↑ 5.0 5.1 "Steroid-protein interactions. Human corticosteroid binding globulin: some physicochemical properties and binding specificity". Biochemistry 20 (21): 6211–8. October 1981. doi:10.1021/bi00524a047. PMID 7306509.
- ↑ 6.0 6.1 "Transport of steroid hormones: binding of 21 endogenous steroids to both testosterone-binding globulin and corticosteroid-binding globulin in human plasma". The Journal of Clinical Endocrinology and Metabolism 53 (1): 58–68. July 1981. doi:10.1210/jcem-53-1-58. PMID 7195404.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 7.8 "Intramuscular administration of hydroxyprogesterone caproate in patients with endometrial carcinoma. Pharmacokinetics and effects on adrenal function". Acta Obstetricia et Gynecologica Scandinavica 64 (6): 519–23. 1985. doi:10.3109/00016348509156732. PMID 2932883.
- ↑ 8.0 8.1 8.2 8.3 8.4 "17 α-hydroxyprogesterone caproate (Makena): a guide to its use in the prevention of preterm birth". Clinical Drug Investigation 33 (3): 223–7. March 2013. doi:10.1007/s40261-013-0060-6. PMID 23413110.
- ↑ 9.00 9.01 9.02 9.03 9.04 9.05 9.06 9.07 9.08 9.09 9.10 9.11 9.12 9.13 "Prevention of preterm delivery with 17-hydroxyprogesterone caproate: pharmacologic considerations". Seminars in Perinatology 38 (8): 516–22. December 2014. doi:10.1053/j.semperi.2014.08.013. PMID 25256193.
- ↑ 10.0 10.1 "FDA Commissioner and Chief Scientist Announce Decision to Withdraw Approval of Makena". U.S. Food and Drug Administration (Press release). 6 April 2023. Archived from the original on 6 April 2023. Retrieved 7 April 2023.
 This article incorporates text from this source, which is in the public domain.
This article incorporates text from this source, which is in the public domain.
- ↑ "Preterm Birth Drug Withdrawn After 12 Years". The New York Times. 8 March 2023. https://www.nytimes.com/2023/03/07/health/preterm-birth-drug-makena-fda.html.
- ↑ "Maker of preterm birth drug Makena to withdraw it from market". 8 March 2023. https://www.cnn.com/2023/03/08/health/makena-preterm-birth-withdrawn-covis/index.html.
- ↑ "Covis Pharma Responds to Presiding Officer's Report Summarizing FDA Advisory Committee Hearing" (Press release). Covis Pharma. 7 March 2023. Archived from the original on 13 March 2023. Retrieved 13 March 2023 – via GlobeNewswire.
- ↑ "17-alpha hydroxyprogesterone caproate for preterm birth prevention: Where have we been, how did we get here, and where are we going?". Seminars in Perinatology 41 (8): 461–467. December 2017. doi:10.1053/j.semperi.2017.08.004. PMID 28947068. https://cdr.lib.unc.edu/downloads/028712685. Retrieved 2 November 2021.
- ↑ 15.0 15.1 "A review of "once-a-month" combined injectable contraceptives". Journal of Obstetrics and Gynaecology 4 (Suppl 1): S1-34. 1994. doi:10.3109/01443619409027641. PMID 12290848.
- ↑ 16.0 16.1 16.2 16.3 16.4 16.5 16.6 16.7 "Progesterone is not the same as 17α-hydroxyprogesterone caproate: implications for obstetrical practice". American Journal of Obstetrics and Gynecology 208 (6): 421–6. June 2013. doi:10.1016/j.ajog.2013.04.027. PMID 23643669.
- ↑ 17.0 17.1 Ovarian Function and its Disorders: Diagnosis and Therapy. Springer Science & Business Media. 6 December 2012. pp. 95–. ISBN 978-94-009-8195-9. https://books.google.com/books?id=7IrpCAAAQBAJ&pg=PA95. Retrieved 16 September 2018.
- ↑ 18.0 18.1 18.2 Steroidal Activity in Experimental Animals and Man. Elsevier Science. 5 December 2016. pp. 398–. ISBN 978-1-4832-7299-3. https://books.google.com/books?id=BbLfBAAAQBAJ&pg=PA398. Retrieved 2 February 2018. "Intramuscular administration of 17α-hydroxyprogesterone caproate produced signs and symptoms of adrenal insufficiency in Addisonians maintained on cortisol and 9α-fluorocortisol (Melby, 1961) and thereby showed properties similar to progesterone and 17α-hydroxyprogesterone. However, further tests will be required to eludicate its pharmacodynamics properties. Contrastingly, there was no evidence for salt dissipation with the test of a smaller dose of the steroid to normal subjects (Landau et al., 1958)."
- ↑ 19.0 19.1 19.2 "Progesterone therapy in pre-eclamptic toxaemia". Acta Obstetricia et Gynecologica Scandinavica 54 (3): 195–202. 1975. doi:10.3109/00016347509157760. PMID 1163210. "Melby (14) found that when progesterone was administered to patients suffering from the syndrome of idiopathic oedema, they experienced a diuresis, with a high excretion of sodium and water within 24 hours after a single injection of 500 mg of 17-α-hydroxyprogesterone caproate.".
- ↑ 20.0 20.1 20.2 20.3 "Treatment of benign prostatic hypertrophy with hydroxyprogesterone caproate: effect on clinical symptoms, morphology, and endocrine function". JAMA 193 (2): 121–8. July 1965. doi:10.1001/jama.1965.03090020035009. PMID 14304354.
- ↑ 21.0 21.1 21.2 21.3 21.4 21.5 "17 hydroxyprogesterone for the prevention of preterm delivery". Obstetrics and Gynecology 105 (5 Pt 1): 1128–35. May 2005. doi:10.1097/01.AOG.0000160432.95395.8f. PMID 15863556.
- ↑ 22.00 22.01 22.02 22.03 22.04 22.05 22.06 22.07 22.08 22.09 22.10 22.11 22.12 22.13 22.14 "Regulation of progesterone signaling during pregnancy: implications for the use of progestins for the prevention of preterm birth". The Journal of Steroid Biochemistry and Molecular Biology 139: 173–81. January 2014. doi:10.1016/j.jsbmb.2013.01.015. PMID 23410596.
- ↑ 23.0 23.1 International Agency for Research on Cancer (1979). Sex Hormones (II).. International Agency for Research on Cancer. p. 401. ISBN 978-92-832-1221-8. https://books.google.com/books?id=nrhrAAAAMAAJ. "17α-Hydroxyprogesterone caproate was first marketed commercially in Japan in 1954-1955."
- ↑ 24.00 24.01 24.02 24.03 24.04 24.05 24.06 24.07 24.08 24.09 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 532–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA532.
- ↑ 25.0 25.1 "The Orphan Drug Act: How the FDA Unlawfully Usurped Market Exclusivity". Northwestern Journal of Technology and Intellectual Property (Heinonline) 11: [v]. http://heinonline.org/HOL/LandingPage?handle=hein.journals%2Fnwteintp11&div=30&id=&page=. Retrieved 18 July 2016.
- ↑ 26.0 26.1 26.2 26.3 26.4 26.5 "Unintended consequences--the cost of preventing preterm births after FDA approval of a branded version of 17OHP". The New England Journal of Medicine 364 (18): 1689–91. May 2011. doi:10.1056/NEJMp1102796. PMID 21410391.
- ↑ SMFM Clinical Guideline: Progesterone and preterm birth prevention: translating clinical trials data into clinical practice, AJOG May 2012
- ↑ 28.0 28.1 28.2 28.3 "Prevention of recurrent preterm delivery by 17 alpha-hydroxyprogesterone caproate". The New England Journal of Medicine 348 (24): 2379–85. June 2003. doi:10.1056/NEJMoa035140. PMID 12802023.
- ↑ "Progesterone and preterm: seventy years of "déjà vu" or "still to be seen"?". Birth 31 (3): 230–5. September 2004. doi:10.1111/j.0730-7659.2004.00315.x. PMID 15330887.
- ↑ "Efficacy of 17alpha-hydroxyprogesterone caproate in the prevention of premature labor". The New England Journal of Medicine 293 (14): 675–80. October 1975. doi:10.1056/nejm197510022931401. PMID 1099445.
- ↑ "Prevention of premature labor by 17 alpha-hydroxyprogesterone caproate". American Journal of Obstetrics and Gynecology 151 (5): 574–7. March 1985. doi:10.1016/0002-9378(85)90141-3. PMID 3976757.
- ↑ "Follow-up of children exposed in utero to 17 alpha-hydroxyprogesterone caproate compared with placebo". Obstetrics and Gynecology 110 (4): 865–72. October 2007. doi:10.1097/01.AOG.0000281348.51499.bc. PMID 17906021.
- ↑ "Advisory Committees: CDER 2006 Meeting Documents". https://www.fda.gov/ohrms/dockets/ac/cder06.html#rhdac.
- ↑ "Embryotoxicity of sex steroidal hormones in nonhuman primates: II. Hydroxyprogesterone caproate, estradiol valerate". Teratology 35 (1): 129–36. February 1987. doi:10.1002/tera.1420350116. PMID 3563931.
- ↑ Duke University Medical Center, New England Journal of Medicine, correspondence, vol 349.
- ↑ "The effect of 17 alpha-hydroxyprogesterone caproate on pregnancy outcome in an active-duty military population". American Journal of Obstetrics and Gynecology 146 (2): 187–90. May 1983. doi:10.1016/0002-9378(83)91051-7. PMID 6682631.
- ↑ "Vaginal progesterone prophylaxis for preterm birth (the OPPTIMUM study): a multicentre, randomised, double-blind trial" (in en). Lancet 387 (10033): 2106–2116. May 2016. doi:10.1016/S0140-6736(16)00350-0. PMID 26921136.
- ↑ "BMJ Blogs: The BMJ » Blog Archive » Richard Lehman's journal review—23 May 2016". 23 May 2016. http://blogs.bmj.com/bmj/2016/05/23/richard-lehmans-journal-review-23-may-2016/.
- ↑ "Prenatal administration of progestogens for preventing spontaneous preterm birth in women with a multiple pregnancy". The Cochrane Database of Systematic Reviews 2019 (11). November 2019. doi:10.1002/14651858.CD012024.pub3. PMID 31745984.
- ↑ "Hydroxyprogesterone caproate therapy in advanced endometrial cancer". Cancer 27 (3): 485–502. March 1971. doi:10.1002/1097-0142(197103)27:3<485::AID-CNCR2820270302>3.0.CO;2-1. PMID 5549492.
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 "Hydroxyprogesterone". https://www.drugs.com/international/hydroxyprogesterone.html.
- ↑ "Therapeutic Regimens". Menopause: Physiology and Pharmacology. Year Book Medical. 1987. pp. 335–351. ISBN 9780815159148. https://books.google.com/books?id=0q1sAAAAMAAJ. Retrieved 3 December 2019.
- ↑ "Efficacy and safety of 17alpha-hydroxyprogesterone caproate in hormone replacement therapy". Gynecological Endocrinology 21 (5): 265–267. November 2005. doi:10.1080/09513590500368650. PMID 16373245.
- ↑ 44.0 44.1 44.2 Benign Prostatic Hypertrophy. Springer Science & Business Media. 6 December 2012. pp. 266–. ISBN 978-1-4612-5476-8. https://books.google.com/books?id=Z5K-BwAAQBAJ&pg=PA266. Retrieved 3 October 2016.
- ↑ 45.0 45.1 Metastasis of Prostate Cancer. Springer Science & Business Media. 5 September 2007. pp. 286–. ISBN 978-1-4020-5847-9. https://books.google.com/books?id=AAo2qVvcVJcC&pg=PA286. Retrieved 23 December 2018.
- ↑ Cancer of the Prostate and Kidney. Springer Science & Business Media. 29 June 2013. pp. 309, 339. ISBN 978-1-4684-4349-3. https://books.google.com/books?id=gtAFCAAAQBAJ&pg=PA309. Retrieved 23 December 2018.
- ↑ 47.0 47.1 Prostatic Carcinoma: Biology and Diagnosis. Springer Science & Business Media. 6 December 2012. pp. 128–. ISBN 978-94-009-8887-3. https://books.google.com/books?id=XNt9BwAAQBAJ&pg=PT128. Retrieved 23 December 2018.
- ↑ 48.0 48.1 48.2 The Treatment of Prostatic Hypertrophy and Neoplasia. Springer Science & Business Media. 9 March 2013. pp. 39,132. ISBN 978-94-015-7190-6. https://books.google.com/books?id=Nc8hBQAAQBAJ&pg=PA39. "Geller has also demonstrated significant decreases in plasma or urine testosterone glucuronide levels following the administration of three other anti-androgens. These include Delalutin [hydroxyprogesterone caproate], chlormadinone acetate, and PH-218. It would appear that decreased androgen production is a property shared by all anti-androgens to date."
- ↑ Meyler's Side Effects of Endocrine and Metabolic Drugs. Elsevier. 21 February 2009. pp. 289–. ISBN 978-0-08-093292-7. https://books.google.com/books?id=BWMeSwVwfTkC&pg=PA289. Retrieved 3 October 2016.
- ↑ Transgender Care: Recommended Guidelines, Practical Information, and Personal Accounts. Temple University Press. 1 March 2001. pp. 58–. ISBN 978-1-56639-852-7. https://books.google.com/books?id=IlPX6E5glDEC&pg=PA58. Retrieved 3 October 2016.
- ↑ The Transgender Phenomenon. SAGE Publications. 23 October 2006. pp. 48–. ISBN 978-1-84787-726-0. https://books.google.com/books?id=2TlvmbN9X7wC&pg=PA48. Retrieved 3 October 2016.
- ↑ Voice and Communication Therapy for The Transgender/Transsexual Client: A Comprehensive Clinical Guide. Plural Publishing. 1 May 2012. pp. 486–. ISBN 978-1-59756-631-5. https://books.google.com/books?id=zVc0BwAAQBAJ&pg=PA486.
- ↑ "Status of sex reassignment surgery for gender identity disorder in Japan". International Journal of Urology 19 (5): 402–414. May 2012. doi:10.1111/j.1442-2042.2012.02975.x. PMID 22372595.
- ↑ "Altered arterial stiffness in male-to-female transsexuals undergoing hormonal treatment". The Journal of Obstetrics and Gynaecology Research 38 (6): 932–940. June 2012. doi:10.1111/j.1447-0756.2011.01815.x. PMID 22487218. http://ousar.lib.okayama-u.ac.jp/48439. Retrieved 18 August 2019.
- ↑ 55.0 55.1 Principles and Practice of Endocrinology and Metabolism. Lippincott Williams & Wilkins. 2001. pp. 757–759, 2168. ISBN 978-0-7817-1750-2. https://books.google.com/books?id=FVfzRvaucq8C&pg=PA757. Retrieved 2 February 2018.
- ↑ Pharmaceutical Dosage Forms - Parenteral Medications, Third Edition: Volume 1: Formulation and Packaging. CRC Press. 19 April 2016. pp. 161–. ISBN 978-1-4200-8644-7. https://books.google.com/books?id=Hkm3BgAAQBAJ&pg=PA161. Retrieved 15 September 2018.
- ↑ 57.0 57.1 European Drug Index: European Drug Registrations (Fourth ed.). CRC Press. 19 June 1998. pp. 561–. ISBN 978-3-7692-2114-5. https://books.google.com/books?id=2HBPHmclMWIC&pg=PA561. Retrieved 15 September 2018.
- ↑ 58.0 58.1 "Pharmacokinetics of once-a-month injectable contraceptives". Contraception 49 (4): 347–59. April 1994. doi:10.1016/0010-7824(94)90032-9. PMID 8013219.
- ↑ 59.0 59.1 59.2 59.3 59.4 "Pharmacodynamic effects of once-a-month combined injectable contraceptives". Contraception 49 (4): 361–385. April 1994. doi:10.1016/0010-7824(94)90033-7. PMID 8013220.
- ↑ 60.0 60.1 "Increasing use of long-acting reversible contraception: safe, reliable, and cost-effective birth control". World J Pharm Pharm Sci 3 (10): 364–392. 2014. ISSN 2278-4357. http://www.wjpps.com/download/article/1412071798.pdf. Retrieved 15 September 2018.
- ↑ 61.0 61.1 61.2 Klinische Endokrinologie für Frauenärzte. Springer-Verlag. 17 April 2013. pp. 533–. ISBN 978-3-662-08110-5. https://books.google.com/books?id=YTiuBgAAQBAJ&pg=PA533. Retrieved 15 September 2018.
- ↑ 62.0 62.1 62.2 62.3 62.4 Lehrbuch der Gynäkologie. Springer-Verlag. 17 April 2013. pp. 214, 255. ISBN 978-3-662-00942-0. https://books.google.com/books?id=ACybBwAAQBAJ&pg=PA214. Retrieved 15 September 2018.
- ↑ 63.0 63.1 63.2 Konservative Therapie der Frauenkrankheiten: Anzeigen, Grenzen und Methoden Einschliesslich der Rezeptur. Springer-Verlag. 8 March 2013. pp. 22–. ISBN 978-3-7091-5694-0. https://books.google.com/books?id=Hte1BgAAQBAJ&pg=PA22. Retrieved 15 September 2018.
- ↑ 64.0 64.1 64.2 Hagers Handbuch der Pharmazeutischen Praxis: Für Apotheker, Arzneimittelhersteller, Drogisten, Ärzte und Medizinalbeamte. Springer-Verlag. 9 March 2013. pp. 1163–. ISBN 978-3-642-49759-9. https://books.google.com/books?id=C_SgBgAAQBAJ&pg=PA1163. Retrieved 15 September 2018.
- ↑ 65.0 65.1 65.2 65.3 65.4 65.5 65.6 65.7 "Die therapeutische Anwendung der Gestagene beim Menschen". Die Gestagene. Handbuch der experimentellen Pharmakologie / Handbook of Experimental Pharmacology. Springer-Verlag. 1968. pp. 1026–1124. doi:10.1007/978-3-642-99941-3_7. ISBN 978-3-642-99941-3. https://books.google.com/books?id=t8GpBgAAQBAJ&pg=PA1045. Retrieved 15 September 2018. "Depotinjektionen [...] 2. Einmalige Injektion von 125mg oder 250mg 17α-Hydroxyprogesteroncapronat als Depotgestagen und 10 mg Oestradiolbenzoat in öliger Lösung (Primosiston) [47, 81, 110, 563, 523, 571, 718, 721, 732, 733, 864, 872, 933, 973, 400]."
- ↑ "EP Hormone - Drugs.com". https://www.drugs.com/international/ep-hormone.html.
- ↑ "Influence of hormones on proliferation of ER-positive cells and ER-negative cells of human breast cancer (MCF-7)". Oncology 47 (1): 19–24. 1990. doi:10.1159/000226779. PMID 2137212. "After the transplantation, each mouse received an intramuscular injection of 0.1 ml EP Hormone Depot consisting of 1 mg/ml 17-β-estradiol dipropionate and 50 mg/ml hydroxyprogesterone caproate every week.".
- ↑ "Fertility promoting and inhibiting effects of new steroid hormonal substances". J Am Med Assoc 169 (16): 1843–54. April 1959. doi:10.1001/jama.1959.03000330015003. PMID 13640942.
- ↑ 69.0 69.1 "What agent should be used to prevent recurrent preterm birth: 17-P or natural progesterone?". Obstetrics and Gynecology Clinics of North America 38 (2): 235–46, ix–x. June 2011. doi:10.1016/j.ogc.2011.02.014. PMID 21575799.
- ↑ 70.0 70.1 Varga A, Henriksen E. Clinical and Histopathologic Evaluation of the Effect of 17-alpha-Hydroxyprogesterone-17-n-caproate on Endometrial Carcinoma. Obstetrics & Gynecology. December 1961. Volume 18. Issue 6. pp. 658-672.
- ↑ 71.0 71.1 "Pharmacology of estrogens and progestogens: influence of different routes of administration". Climacteric 8 (Suppl 1): 3–63. August 2005. doi:10.1080/13697130500148875. PMID 16112947. http://hormonebalance.org/images/documents/Kuhl%2005%20%20Pharm%20Estro%20Progest%20Climacteric_1313155660.pdf. Retrieved 25 March 2018.
- ↑ 72.0 72.1 Geburtshilfe und Gynäkologie: Physiologie und Pathologie der Reproduktion. Springer-Verlag. 8 March 2013. pp. 583–. ISBN 978-3-642-95583-9. https://books.google.com/books?id=tpmgBgAAQBAJ&pg=PA583. Retrieved 29 March 2019.
- ↑ "Clinical use of oestrogens and progestogens". Maturitas 12 (3): 199–214. September 1990. doi:10.1016/0378-5122(90)90004-P. PMID 2215269.
- ↑ 74.0 74.1 "Existing once-a-month combined injectable contraceptives". Contraception 49 (4): 293–301. April 1994. doi:10.1016/0010-7824(94)90029-9. PMID 8013216.
- ↑ 75.0 75.1 "Conception control by long-acting progestogens: preliminary report". Obstetrics and Gynecology 21: 666–8. June 1963. PMID 13992789. https://journals.lww.com/greenjournal/Citation/1963/06000/Conception_Control_by_Long_acting_Progestogens_.3.aspx. Retrieved 12 February 2020.
- ↑ "The use of progestational agents in obstetrics and gynecology". Clinical Obstetrics and Gynecology 3 (4): 1047–67. December 1960. doi:10.1097/00003081-196003040-00019. PMID 13756432. "50 mg of [medroxyprogesterone acetate], intramuscularly, is equivalent to 250 mg [hydroxyprogesterone caproate]".
- ↑ "Depo_Provera". 2016. https://www.accessdata.fda.gov/drugsatfda_docs/label/2016/020246s058lbl.pdf.
- ↑ 78.0 78.1 78.2 78.3 78.4 78.5 78.6 78.7 "Comparison of progesterone and glucocorticoid receptor binding and stimulation of gene expression by progesterone, 17-alpha hydroxyprogesterone caproate, and related progestins". American Journal of Obstetrics and Gynecology 197 (6): 599.e1–7. December 2007. doi:10.1016/j.ajog.2007.05.024. PMID 18060946.
- ↑ "[Changes in reproductive hormones levels in the treatment of endometrial precancerous lesion with hydroxyprogesterone caproate]" (in zh). Zhonghua Fu Chan Ke Za Zhi 29 (4): 205–6, 251. April 1994. PMID 8082440. "In this paper, 14 cases of precancerous lesion of endometrium were treated with hydroxyprogesterone caproate and a series of hormone determination was analysed before and after treatment. Results showed that LH and LH/FSH were dramatically decreased. (LH P < 0.05, LH/FSH P < 0.01).".
- ↑ Benign Prostatic Hypertrophy. Springer Science & Business Media. 6 December 2012. pp. 266–. ISBN 978-1-4612-5476-8. https://books.google.com/books?id=Z5K-BwAAQBAJ&pg=PA266. Retrieved 3 October 2016. "Since the initial report by Geller and associates28 on the use of hydroxyprogesterone caproate in the treatment of BPH, a variety of progestins have been studied in the medical management of this disease: hydroxyprogesterone caproate, chlormadinone acetate,27 and medrogestone (6-methyl-6-dehydro-17-methylprogesterone).50 These drugs should have a beneficial effect in BPH as they inhibit testicular function by suppressing serum LH and have no intrinsic estrogenic or androgenic activity."
- ↑ "Treatment of benign prostatic hyperplasia with hydroxyprogesterone-caproate: placebo-controlled study". Urology 9 (2): 144–8. February 1977. doi:10.1016/0090-4295(77)90184-4. PMID 65818.
- ↑ "Effect of progestational agents on carcinoma of the prostate". Cancer Chemother Rep 51 (1): 41–6. February 1967. PMID 6039663.
- ↑ "Progesterone treatment for local recurrence and metastases in carcinoma corporis uteri". Acta Radiologica 10 (2): 187–92. April 1971. doi:10.3109/02841867109129755. PMID 5556820. "The preparations used were Proluton Depot (17a-hydroxy-progesterone caproate) and in 3 patients SH 5132 (17a-hydroxy-19-norprogesterone caproate); 100 mg of the latter corresponds to 1000 mg of Proluton Depot.".
- ↑ 84.0 84.1 "Short-term progestogen treatment of endometrial carcinoma. Histological, histochemical and hormonal studies". Acta Obstetricia et Gynecologica Scandinavica 51 (1): 55–62. 1972. doi:10.3109/00016347209154968. PMID 4261828.
- ↑ 85.0 85.1 85.2 "On gestagen treatment of advanced prostatic carcinoma". Scandinavian Journal of Urology and Nephrology 12 (2): 119–21. 1978. doi:10.3109/00365597809179977. PMID 694436.
- ↑ 86.0 86.1 "Effects of norgestrel and ethinyloestradiol ingestion on serum levels of sex hormones and gonadotrophins in men". Clinical Endocrinology 11 (5): 497–504. November 1979. doi:10.1111/j.1365-2265.1979.tb03102.x. PMID 519881. "Another synthetic gestogen, 17-hydroxy-19-norprogesterone caproate (Depostat-Schering), 400 mg by i.m. weekly injections suppressed T levels to 25% of pretreatment values (Sander er al., 1978).".
- ↑ Campbell-Walsh Urology: Expert Consult Premium Edition: Enhanced Online Features and Print, 4-Volume Set. Elsevier Health Sciences. 25 August 2011. pp. 2938–. ISBN 978-1-4160-6911-9. https://books.google.com/books?id=fu3BBwAAQBAJ&pg=PA2938. Retrieved 23 December 2018.
- ↑ "Effect of flutamide or cyproterone acetate on pituitary and testicular hormones in normal men". The Journal of Clinical Endocrinology and Metabolism 59 (5): 963–9. November 1984. doi:10.1210/jcem-59-5-963. PMID 6237116.
- ↑ "Treatment of advanced prostatic cancer with parenteral cyproterone acetate: a phase III randomised trial". British Journal of Urology 52 (3): 208–15. June 1980. doi:10.1111/j.1464-410x.1980.tb02961.x. PMID 7000222.
- ↑ "An endocrine basis for endometrial carcinoma". American Journal of Obstetrics and Gynecology 77 (2): 233–42. February 1959. doi:10.1016/0002-9378(59)90223-6. PMID 13617315.
- ↑ The Hypothalamic-Pituitary-Adrenal Axis in Health and Disease: Cushing's Syndrome and Beyond. Springer. 1 December 2016. pp. 28–. ISBN 978-3-319-45950-9. https://books.google.com/books?id=Yw2kDQAAQBAJ&pg=PA28. Retrieved 2 February 2018.
- ↑ Drug Therapy in Nursing. Lippincott Williams & Wilkins. 2009. pp. 674–. ISBN 978-0-7817-6587-9. https://books.google.com/books?id=5zd_W_PUwvYC&pg=PA674. Retrieved 2 February 2018.
- ↑ "Discovery of selective glucocorticoid receptor modulators by multiplexed reporter screening". Proceedings of the National Academy of Sciences of the United States of America 106 (12): 4929–34. March 2009. doi:10.1073/pnas.0812308106. PMID 19255438. Bibcode: 2009PNAS..106.4929G.
- ↑ 94.0 94.1 "Progestins can mimic, inhibit and potentiate the actions of androgens". Pharmacology & Therapeutics 23 (3): 443–59. 1983. doi:10.1016/0163-7258(83)90023-2. PMID 6371845.
- ↑ 95.0 95.1 95.2 "Delalutin and estrogens for the treatment of advanced mammary carcinoma in the postmenopausal woman". Cancer 18 (4): 436–446. April 1965. doi:10.1002/1097-0142(196504)18:4<436::AID-CNCR2820180407>3.0.CO;2-D. PMID 14278040.
- ↑ 96.0 96.1 "The effect of progesterone on myometrial contractility, potassium channels, and tocolytic efficacy". Reproductive Sciences 16 (11): 1052–61. November 2009. doi:10.1177/1933719109340926. PMID 19602723.
- ↑ Reproduction in Domestic Animals. Elsevier. 20 February 1991. pp. 101–. ISBN 978-0-08-057109-6. https://books.google.com/books?id=bbb-ow0N7K4C&pg=PA101.
- ↑ 98.0 98.1 "Non-genomic progesterone actions in female reproduction". Human Reproduction Update 15 (1): 119–38. 2009. doi:10.1093/humupd/dmn044. PMID 18936037.
- ↑ 99.0 99.1 "Revisiting the roles of progesterone and allopregnanolone in the nervous system: resurgence of the progesterone receptors". Progress in Neurobiology 113: 6–39. February 2014. doi:10.1016/j.pneurobio.2013.09.004. PMID 24172649.
- ↑ "The importance of 'awareness' for understanding fetal pain". Brain Research. Brain Research Reviews 49 (3): 455–71. November 2005. doi:10.1016/j.brainresrev.2005.01.006. PMID 16269314.
- ↑ "The emergence of human consciousness: from fetal to neonatal life". Pediatric Research 65 (3): 255–60. March 2009. doi:10.1203/PDR.0b013e3181973b0d. PMID 19092726. https://philpapers.org/rec/LAGTEO-5. Retrieved 18 August 2019. "[...] the fetus is sedated by the low oxygen tension of the fetal blood and the neurosteroid anesthetics pregnanolone and the sleep-inducing prostaglandin D2 provided by the placenta (36).".
- ↑ 102.0 102.1 102.2 "Pharmacology and placental transport of 17-hydroxyprogesterone caproate in singleton gestation". American Journal of Obstetrics and Gynecology 207 (5): 398.e1–8. November 2012. doi:10.1016/j.ajog.2012.08.015. PMID 22967833.
- ↑ 103.0 103.1 "Pharmacokinetics of 17-hydroxyprogesterone caproate in multifetal gestation". American Journal of Obstetrics and Gynecology 205 (1): 40.e1–8. July 2011. doi:10.1016/j.ajog.2011.03.028. PMID 21620357.
- ↑ "Orally Active Progestational Compounds. Human Studies: Effects on the Utero-Vaginal Tract". Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. II. Pergamon Press. September 1972. pp. 245–273. ISBN 978-0080168128. OCLC 278011135. https://books.google.com/books?id=Nv5sAAAAMAAJ.
- ↑ "Observations of the role of progestational agents in human gynecologic disorders and pregnancy complications". Ann. N. Y. Acad. Sci. 71 (5): 727–52. July 1958. doi:10.1111/j.1749-6632.1958.tb46803.x. PMID 13583829. Bibcode: 1958NYASA..71..727B.
- ↑ "Die perorale wirksamkeit synthetischer gestagene" (in de). Zentralbl Gynakol 79 (14): 529–39. April 1957. PMID 13443471.
- ↑ 107.0 107.1 107.2 "Hydroxyprogesterone caproate oral - Lipocine - AdisInsight". https://adisinsight.springer.com/drugs/800038483.
- ↑ 108.0 108.1 "770: Pharmacokinetics and tolerability of oral 17-hydroxyprogesterone caproate (HPC) relative to intramuscular (IM) HPC.". American Journal of Obstetrics & Gynecology 212 (1): S374. January 2015. doi:10.1016/j.ajog.2014.10.976.
- ↑ "Pharmacokinetics of Hydroxyprogesterone Caproate and its Primary Metabolites during Pregnancy". AJP Reports 8 (2): e106–e112. April 2018. doi:10.1055/s-0038-1639331. PMID 29765789.
- ↑ 110.0 110.1 "Highlight of Prescribing Information: MAKENATM (hydroxyprogesterone caproate injection) for intramuscular use.". U.S. Food and Drug Administration. February 2011. https://www.accessdata.fda.gov/drugsatfda_docs/label/2011/021945s000lbl.pdf.
- ↑ 111.0 111.1 "Effects, Duration of Action and Metabolism in Man". Pharmacology of the Endocrine System and Related Drugs: Progesterone, Progestational Drugs and Antifertility Agents. II. Pergamon Press. September 1972. pp. 13–24. ISBN 978-0080168128. OCLC 278011135. https://books.google.com/books?id=Nv5sAAAAMAAJ.
- ↑ 112.0 112.1 "Treatment of persistent acne in women with 17 alpha hydroxyprogesterone caproate (delalutin); a preliminary report". The Journal of Investigative Dermatology 31 (5): 247–50. November 1958. doi:10.1038/jid.1958.114. PMID 13598928.
- ↑ 113.0 113.1 113.2 113.3 113.4 113.5 113.6 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 664–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA664.
- ↑ Pharmaceutical Substances, 5th Edition, 2009: Syntheses, Patents and Applications of the most relevant APIs. Thieme. 14 May 2014. pp. 677–679. ISBN 978-3-13-179275-4. https://books.google.com/books?id=4lCGAwAAQBAJ&pg=PA677. Retrieved 6 September 2018.
- ↑ William Andrew Publishing (22 October 2013). Pharmaceutical Manufacturing Encyclopedia. Elsevier. pp. 1865–1866. ISBN 978-0-8155-1856-3. https://books.google.com/books?id=_J2ti4EkYpkC&pg=PA1865. Retrieved 6 September 2018.
- ↑ "Chemie der Gestagene". Die Gestagene. Handbuch der experimentellen Pharmakologie / Handbook of Experimental Pharmacology. Springer-Verlag. 1968. pp. 1–44. doi:10.1007/978-3-642-99941-3_1. ISBN 978-3-642-99941-3. https://books.google.com/books?id=t8GpBgAAQBAJ&pg=PA1045. Retrieved 15 September 2018. "3. Hydroxyprogesteron-caproat. C27H4004, Mol.-Gew.: 428,62; chemische Bezeichnung Δ4-Pregnen-17α-ol-3,20-dion-17α-capronat, Trivialnamen: Hydroxyprogesteroncapronat, 17α-Hydroxyprogesteron-17α-capronat. Synthese: [88]. Darstellung: [88]. Eigenschaften: weißes kristallines Pulver (aus Isopropyläther) oder Methanol, F.: 119-122⁰, [α]D: +60⁰ (Chlf.) UV-Absorption: λmax.: 240 mμ, ε = 17000. Dipolmoment: [μ = 2,21 (Benzol). Leicht löslich in Äthanol, Äther, Essigester, Benzol, Chloroform, löslich in: Petroläther, unlöslich in Wasser. Bei 20⁰ lösen 100 ml Sesamöl ca. 4,0 g, Ricinusöl ca. 2,5 g, Ricinusöl: Benzylbenzoat (4: 6) ca. 26,5 g, Benzylbenzoat ca. 36,0 g. [...] Abb. 3. IR-Spektrum [126] und Formel des Hydroxyprogesteron-caproat."
- ↑ "Über protrahiert wirksame Gestagene". Naunyn-Schmiedebergs Archiv für Experimentelle Pathologie und Pharmakologie 223 (3). 1954. doi:10.1007/BF00246995.
- ↑ "Comparative activity of progestational agents on the human endometrium and vaginal epithelium of surgical castrates". Annals of the New York Academy of Sciences 71 (5): 599–616. July 1958. doi:10.1111/j.1749-6632.1958.tb46791.x. PMID 13583817. Bibcode: 1958NYASA..71..599W. "In the group of new parenteral progestational agents, three substances developed by Karl Junkmann1,2 are the most outstanding and interesting: 17a-hydroxyprogesterone caproate and 17a-hydroxyprogesterone acetate, introduced in 1953, and the most potent of all new parenteral progestational agents, 17-ethynyl-19-nortestosterone enanthate, introduced in 1956.".
- ↑ ACRH. U.S. Dept. of Energy. 1960. p. 71. https://books.google.com/books?id=HQVYAAAAYAAJ. "[The] minimal activity [of 17(a)-hydroxyprogesterone] is magnified to an unexpected degree by the esterification of this steroid with caproic acid to produce 17(a)-hydroxyprogesterone-17-n-caproate, first reported by Karl Junkmann in 1954.6,7"
- ↑ Methods in Hormone Research. Academic Press. 1966. p. 86. https://books.google.com/books?id=mQVHAAAAYAAJ. "Junkmann (1954) reported that the acetate, butyrate, and caproate forms had both increased and prolonged activity, [...]"
- ↑ Steroid Drugs. Blakiston Division, McGraw-Hill. 1962. pp. 101–102. https://books.google.com/books?id=SIhLAAAAYAAJ. "Junkmann of Schering, AG., however, was able to show that long chain esters of 17a-hydroxyprogesterones such as the 17a-caproate produced powerful long-acting progestational effect. This compound is marketed in the United States as Delalutin by Squibb, and has been heavily used for the treatment of habitual abortion."
- ↑ New and Nonofficial Drugs. Lippincott. 1958. p. 662. https://books.google.com/books?id=eY4wAAAAIAAJ. "Supplied by.—E. R. Squibb & Sons (Delalutin). Year of introduction: 1956."
- ↑ Pharmacology of hormones. Thieme. 1975. p. 105. ISBN 978-3-13-518901-7. https://books.google.com/books?id=9WhNAQAAIAAJ. "Progesterone itself is now almost never used for the management of any imminent threat to pregnancy. For oral therapy, it is in any event unsuitable and for injections, it has now been replaced by the long-acting esters of 17α-hydroxyprogesterone. The caproate (Proluton, Delalutin), a long-acting ester, is available in [...] Progesterone is rarely used therapeutically. It has largely been superseded by a long-acting ester of 17α-hydroxyprogesterone, for parenteral therapy."
- ↑ "FDA press release regarding Makena approval". https://www.fda.gov/NewsEvents/Newsroom/PressAnnouncements/ucm242234.htm.
- ↑ "Accelerated Approval Program". 30 January 2023. https://www.fda.gov/drugs/nda-and-bla-approvals/accelerated-approval-program.
- ↑ "17-OHPC to Prevent Recurrent Preterm Birth in Singleton Gestations (PROLONG Study): A Multicenter, International, Randomized Double-Blind Trial". American Journal of Perinatology 37 (2): 127–136. January 2020. doi:10.1055/s-0039-3400227. PMID 31652479.
- ↑ "CDER proposes withdrawal of approval for Makena". 5 October 2020. https://www.fda.gov/drugs/drug-safety-and-availability/cder-proposes-withdrawal-approval-makena.
- ↑ "Medication safety is still an issue in obstetrics 50 years after the Kefauver-Harris amendments: the case of progestogens". Ultrasound in Obstetrics & Gynecology 42 (3): 247–53. September 2013. doi:10.1002/uog.12456. PMID 23495199.
- ↑ 129.0 129.1 129.2 "Sex hormones and their modulators". Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. 2009. pp. 2110–2111. ISBN 978-0-85369-840-1.
- ↑ "Macleans.ca - Canada's national current affairs and news magazine since 1905". http://www.macleans.ca/article.jsp?content=w6411446.
- ↑ "Price of preterm birth medicine cut". Associated Press. Boston.com. 2 April 2011. http://www.boston.com/news/nation/washington/articles/2011/04/02/price_of_preterm_birth_medicine_cut/.
- ↑ "Compounded Drug Products That Are Essentially Copies of a Commercially Available Drug Product Under Section 503A of the Federal Food, Drug, and Cosmetic Act Guidance for Industry". 1 April 2019. https://www.fda.gov/regulatory-information/search-fda-guidance-documents/compounded-drug-products-are-essentially-copies-commercially-available-drug-product-under-section.
- ↑ "FDA to Restrict Compounders from Making Copies of Commercially Available Drugs". 7 July 2016. https://www.raps.org/regulatory-focus%E2%84%A2/news-articles/2016/7/fda-to-restrict-compounders-from-making-copies-of-commercially-available-drugs.
- ↑ Antibiotic Medicine and Clinical Therapy. 1959. p. 249. https://books.google.com/books?id=Q6pKAQAAIAAJ.
- ↑ "Monthly Injectable Contraceptives". Long-Acting Contraception. 1983. pp. 93–103. OCLC 35018604. https://scholar.google.com/scholar?cluster=14664537528797672080. Retrieved 27 December 2019.
- ↑ "The clinical use of monthly injectable contraceptive preparations". Obstetrical & Gynecological Survey 32 (6): 335–47. June 1977. doi:10.1097/00006254-197706000-00001. PMID 865726.
- ↑ "Pharmacology and Clinical Utility of Hormones in Hormone Related Neoplasms". Antineoplastic and Immunosuppressive Agents. Handbuch der experimentellen Pharmakologie / Handbook of Experimental Pharmacology. Springer. 1975. pp. 170–192. doi:10.1007/978-3-642-65806-8_11. ISBN 978-3-642-65806-8. https://books.google.com/books?id=aU_oCAAAQBAJ&pg=PA170. Retrieved 31 May 2019.
- ↑ "Clinical trial of delautin in the treatment of advanced mammary carcinoma in postmenopausal women". Cancer 15 (6): 1218–1220. 1962. doi:10.1002/1097-0142(196211/12)15:6<1218::AID-CNCR2820150619>3.0.CO;2-Y. PMID 14024037.
- ↑ "Objective remission of metastatic breast carcinoma in a male who received 17-alpha hydroxy progesterone caproate (delalutin)". Cancer Chemotherapy Reports 14: 77–81. October 1961. PMID 13897631.
- ↑ "Delalutin und Östrogene als Behandlung des vorgeschrittenen Mammakarzinoms bei Frauen nach der Menopause". Gynäkologisch-geburtshilfliche Rundschau 3 (4): 271–272. 1966. doi:10.1159/000266855. ISSN 1018-8843.
- ↑ "[Additive treatment of metastasizing breast cancer with special reference to postmenopausal age (results of a randomized study)]" (in de). Strahlentherapie 152 (3): 235–247. September 1976. PMID 968923.
- ↑ Santhosh CR (2006). Pharmacokinetics of 17α-Hydroxy Progesterone Caproate in Cattle, Buffalo, Sheep and Goat (Ph.D. thesis). Karnataka Veterinary, Animal and Fisheries Sciences University, Bidar. Archived from the original on 15 December 2018.
Further reading
- "17 hydroxyprogesterone for the prevention of preterm delivery". Obstetrics and Gynecology 105 (5 Pt 1): 1128–35. May 2005. doi:10.1097/01.AOG.0000160432.95395.8f. PMID 15863556.
- "Pharmacological use of progesterone and 17-alpha-hydroxyprogesterone caproate in the prevention of preterm delivery". Minerva Ginecologica 61 (5): 401–9. October 2009. PMID 19749671.
- "17 α-Hydroxyprogesterone caproate (Makena™): in the prevention of preterm birth". Paediatric Drugs 13 (5): 337–45. October 2011. doi:10.2165/11208140-000000000-00000. PMID 21888448.
- "17α Hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth". Reproductive Toxicology 33 (1): 15–9. January 2012. doi:10.1016/j.reprotox.2011.10.017. PMID 22120850.
- "The safety of progesterone and 17-hydroxyprogesterone caproate administration for the prevention of preterm birth: an evidence-based assessment". American Journal of Perinatology 29 (9): 665–72. October 2012. doi:10.1055/s-0032-1316444. PMID 22773279.
- "Progesterone is not the same as 17α-hydroxyprogesterone caproate: implications for obstetrical practice". American Journal of Obstetrics and Gynecology 208 (6): 421–6. June 2013. doi:10.1016/j.ajog.2013.04.027. PMID 23643669.
- "Prevention of preterm delivery with 17-hydroxyprogesterone caproate: pharmacologic considerations". Seminars in Perinatology 38 (8): 516–22. December 2014. doi:10.1053/j.semperi.2014.08.013. PMID 25256193.
- "17-alpha-hydroxyprogesterone caproate for maintenance tocolysis: a systematic review and metaanalysis of randomized trials". American Journal of Obstetrics and Gynecology 213 (1): 16–22. July 2015. doi:10.1016/j.ajog.2015.01.054. PMID 25659469.
- "Prevention of preterm birth with vaginal progesterone or 17-alpha-hydroxyprogesterone caproate: a critical examination of efficacy and safety". American Journal of Obstetrics and Gynecology 214 (1): 45–56. January 2016. doi:10.1016/j.ajog.2015.10.934. PMID 26558340.
- "What we have learned about the role of 17-alpha-hydroxyprogesterone caproate in the prevention of preterm birth". Seminars in Perinatology 40 (5): 273–80. August 2016. doi:10.1053/j.semperi.2016.03.002. PMID 27105940.
- "Vaginal progesterone vs intramuscular 17α-hydroxyprogesterone caproate for prevention of recurrent spontaneous preterm birth in singleton gestations: systematic review and meta-analysis of randomized controlled trials". Ultrasound in Obstetrics & Gynecology 49 (3): 315–321. March 2017. doi:10.1002/uog.17245. PMID 27546354. https://touroscholar.touro.edu/nymc_fac_pubs/863.
- "Meta-analysis of randomized controlled trials comparing 17α-hydroxyprogesterone caproate and vaginal progesterone for the prevention of recurrent spontaneous preterm delivery". International Journal of Gynaecology and Obstetrics 138 (1): 12–16. July 2017. doi:10.1002/ijgo.12166. PMID 28369874.
- "17-alpha hydroxyprogesterone caproate for preterm birth prevention: Where have we been, how did we get here, and where are we going?". Seminars in Perinatology 41 (8): 461–467. December 2017. doi:10.1053/j.semperi.2017.08.004. PMID 28947068. https://cdr.lib.unc.edu/downloads/028712685.
 |
![OHPC levels after a single intramuscular injection of 1,000 mg OHPC in five women with endometrial cancer.[7]](/wiki/images/thumb/d/df/Hydroxyprogesterone_caproate_levels_after_a_single_intramuscular_injection_of_1%2C000_mg_hydroxyprogesterone_caproate_in_women.png/447px-Hydroxyprogesterone_caproate_levels_after_a_single_intramuscular_injection_of_1%2C000_mg_hydroxyprogesterone_caproate_in_women.png)
![OHPC levels over the course of a month after a final dose following continuous therapy with 250 mg per week OHPC by intramuscular injection in pregnant women with singleton gestation.[102]](/wiki/images/thumb/b/b2/Hydroxyprogesterone_caproate_levels_after_a_final_dose_following_continuous_therapy_with_250_mg_per_week_by_intramuscular_injection_in_pregnant_women.png/461px-Hydroxyprogesterone_caproate_levels_after_a_final_dose_following_continuous_therapy_with_250_mg_per_week_by_intramuscular_injection_in_pregnant_women.png)
