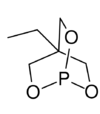
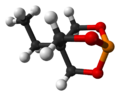
Chemistry:Trimethylolpropane phosphite
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
4-Ethyl-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane | |||
| Other names
EtCage; Ethyl bicyclic phosphite; Trishydroxymethylpropane bicyclic phosphite
| |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| UNII | |||
| |||
| |||
| Properties | |||
| C6H11O3P | |||
| Molar mass | 162.125 g·mol−1 | ||
| Appearance | white waxy solid | ||
| Melting point | 56 °C (133 °F; 329 K) | ||
| organic solvents | |||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trimethylolpropane phosphite, C2H5C(CH2O)3P, is a phosphite ester used as a ligand in organometallic chemistry. Trimethylolpropane phosphite is sometimes abbreviated to EtCage. It is a white solid that is soluble in organic solvents. It is also highly toxic.[1]
Preparation and reactions
It is prepared by reaction of trimethylolpropane with phosphorus trichloride or by transesterification with trimethylphosphite:[2]
- P(OMe)3 + EtC(CH2OH)3 → 3 MeOH + EtC(CH2O)3P
The first member of this series was derived from trimethylolethane,[3] but these derivatives are often poorly soluble. For this reason, the ethyl derivative has received more attention.[4]
Reactions
The compound forms an isolable ozonide, which degrades above 0 °C to release singlet O2.[1]
Coordination chemistry
Several EtCage complexes are known, since the ligand is highly basic (for a phosphite) and has a small ligand cone angle (101°). Illustrative complexes include [(EtCage)2Mo(CO)4], [Ir4(CO)11(EtCage)] and (CpMe5)RuCl(EtCage)2, shown below.
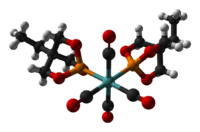 180px|Ball-and-stick model of the (trimethylolpropane phosphite)tetrairidium undecacarbonyl cluster
180px|Ball-and-stick model of the (trimethylolpropane phosphite)tetrairidium undecacarbonyl cluster 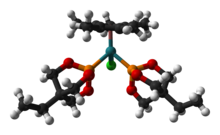
Safety
Trimethylolpropane phosphite is very toxic and is a convulsant. LD50 is 1.1 mg per kg bodyweight (mice, i.p.).[5][6]
References
- ↑ 1.0 1.1 "4-Ethyl-2,6,7-trioxa-1-phosphabicyclo[2.2.2]octane". e-EROS Encyclopedia of Reagents for Organic Synthesis. 2014. pp. 1–3. doi:10.1002/047084289X.rn01781. ISBN 9780470842898.
- ↑ Verkade, J. G. "Spectroscopic studies of metal-phosphorus bonding in coordination complexes" Coordination Chemistry Reviews 1972, vol. 9, 1-106. doi:10.1016/S0010-8545(00)80224-6
- ↑ Verkade, J. G.; Reynolds, L. T. "The synthesis of a novel ester of phosphorus and of arsenic" Journal of Organic Chemistry (1960), 25, 663-5. doi:10.1021/jo01074a622
- ↑ Huttemann, T. J., Jr.; Foxman, B. M.; Sperati, C. R.; Verkade, J. G. "Transition metal complexes of a constrained phosphite ester. IV. Compounds of cobalt(I), cobalt(III), nickel(II), and nickel(0)" Inorganic Chemistry (1965), 4(7), 950-3. doi:10.1021/ic50029a005
- ↑ Ralf Stöhr et al. Chemische Kampfstoffe und Schutz vor chemischen Kampfstoffen 2. Aufl. Militärverlag der DDR, 1985 (german)
- ↑ Milbrath, Dean S.; Engel, Judith L.; Verkade, John G.; Casida, John E. (1979). "Structure-toxicity relationships of 1-substituted-4-alkyl-2,6,7-trioxabicyclo[2.2.2.]octanes". Toxicology and Applied Pharmacology 47 (2): 287–93. doi:10.1016/0041-008X(79)90323-5. PMID 452023.
{{Navbox | name = GABA receptor modulators | title = GABA receptor modulators | state = collapsed | bodyclass = hlist | groupstyle = text-align:center;
| group1 = Ionotropic | list1 = {{Navbox|subgroup | groupstyle = text-align:center | groupwidth = 5em
| group1 = GABAA | list1 =
- Agonists: (+)-Catechin
- Bamaluzole
- Barbiturates (e.g., phenobarbital)
- BL-1020
- DAVA
- Dihydromuscimol
- GABA
- Gabamide
- GABOB
- Gaboxadol (THIP)
- Homotaurine (tramiprosate, 3-APS)
- Ibotenic acid
- iso-THAZ
- iso-THIP
- Isoguvacine
- Isomuscimol
- Isonipecotic acid
- Kojic amine
- Lignans (e.g., honokiol)
- Methylglyoxal
- Monastrol
- Muscimol
- Nefiracetam
- Neuroactive steroids (e.g., allopregnanolone)
- Org 20599
- PF-6372865
- Phenibut
- Picamilon
- P4S
- Progabide
- Propofol
- Quisqualamine
- SL-75102
- TACA
- TAMP
- Terpenoids (e.g., borneol)
- Thiomuscimol
- Tolgabide
- ZAPA
- Positive modulators (abridged; see here for a full list): α-EMTBL
- Alcohols (e.g., ethanol)
- Anabolic steroids
- Avermectins (e.g., ivermectin)
- Barbiturates (e.g., phenobarbital)
- Benzodiazepines (e.g., diazepam)
- Bromide compounds (e.g., potassium bromide)
- Carbamates (e.g., meprobamate)
- Carbamazepine
- Chloralose
- Chlormezanone
- Clomethiazole
- Dihydroergolines (e.g., ergoloid (dihydroergotoxine))
- Etazepine
- Etifoxine
- Fenamates (e.g., mefenamic acid)
- Flavonoids (e.g., apigenin, hispidulin)
- Fluoxetine
- Flupirtine
- Imidazoles (e.g., etomidate)
- Kava constituents (e.g., kavain)<!--PMID: 9776662-->
- Lanthanum
- Loreclezole
- Monastrol
- Neuroactive steroids (e.g., allopregnanolone, [[Chemistry:Cholecholesterol]], THDOC)
- Niacin
- Nicotinamide (niacinamide)
- Nonbenzodiazepines (e.g., β-carbolines (e.g., [[abecarnil), cyclopyrrolones (e.g., zopiclone), imidazopyridines (e.g., zolpidem), pyrazolopyrimidines (e.g., zaleplon))
- Norfluoxetine
- Petrichloral
- Phenols (e.g., propofol)
- Phenytoin
- Piperidinediones (e.g., glutethimide)
- Propanidid
- Pyrazolopyridines (e.g., etazolate)
- Quinazolinones (e.g., methaqualone)
- Retigabine (ezogabine)
- ROD-188
- Skullcap constituents (e.g., baicalin)
- Stiripentol
- Sulfonylalkanes (e.g., sulfonmethane (sulfonal))
- Topiramate
- Valerian constituents (e.g., valerenic acid)
- Volatiles/gases (e.g., chloral hydrate, chloroform, [[Chemistry:Diethyl diethyl ether, Parparaldehyde]], sevoflurane)
- Antagonists: Bicuculline
- Coriamyrtin
- Dihydrosecurinine
- Gabazine (SR-95531)
- Hydrastine
- Hyenachin (mellitoxin)
- PHP-501
- Pitrazepin
- Securinine
- Sinomenine
- SR-42641
- SR-95103
- Thiocolchicoside
- Tutin
- Negative modulators: 1,3M1B
- 3M2B
- 11-Ketoprogesterone
- 17-Phenylandrostenol
- α5IA (LS-193,268)
- β-CCB
- β-CCE
- β-CCM
- β-CCP
- β-EMGBL
- Anabolic steroids
- Amiloride
- Anisatin
- β-Lactams (e.g., penicillins, cephalosporins, carbapenems)
- Basmisanil
- Bemegride
- Bicyclic phosphates (TBPS, TBPO, IPTBO)
- BIDN
- Bilobalide
- Bupropion
- CHEB
- Chlorophenylsilatrane
- Cicutoxin
- Cloflubicyne
- Cyclothiazide
- DHEA
- DHEA-S
- Dieldrin
- (+)-DMBB
- DMCM
- DMPC
- EBOB
- Etbicyphat
- FG-7142 (ZK-31906)
- Fiproles (e.g., fipronil)
- Flavonoids (e.g., amentoflavone, oroxylin A)
- Flumazenil
- Fluoroquinolones (e.g., ciprofloxacin)
- Flurothyl
- Furosemide
- Golexanolone
- Iomazenil (123I)
- IPTBO
- Isopregnanolone (sepranolone)
- L-655,708
- Laudanosine
- Leptazol
- Lindane
- MaxiPost
- Morphine
- Morphine-3-glucuronide
- MRK-016
- Naloxone
- Naltrexone
- Nicardipine
- Nonsteroidal antiandrogens (e.g., [[apalutamide, [[Chemistry:Bicalutbicalutamide, Enzalutenzalutamide, Chemistry:Flutamide|flut]]amide]], nilutamide)
- Oenanthotoxin
- Pentylenetetrazol (pentetrazol)
- Phenylsilatrane
- Picrotoxin (i.e., picrotin, picrotoxinin and dihydropicrotoxinin)
- Pregnenolone sulfate
- Propybicyphat
- PWZ-029
- Radequinil
- Ro 15-4513
- Ro 19-4603
- RO4882224
- RO4938581
- Sarmazenil
- SCS
- Suritozole
- TB-21007
- TBOB
- TBPS
- TCS-1105
- Terbequinil
- TETS
- Thujone
- U-93631
- Zinc
- ZK-93426
| group2 = GABAA-ρ | list2 =
- Agonists: BL-1020
- CACA
- CAMP
- Homohypotaurine
- GABA
- GABOB
- Ibotenic acid
- Isoguvacine
- Muscimol
- N4-Chloroacetylcytosine arabinoside
- Picamilon
- Progabide
- TACA
- TAMP
- Thiomuscimol
- Tolgabide
- Positive modulators: Allopregnanolone
- Alphaxolone
- ATHDOC
- Lanthanides
- Antagonists: (S)-2-MeGABA
- (S)-4-ACPBPA
- (S)-4-ACPCA
- 2-MeTACA
- 3-APMPA
- 4-ACPAM
- 4-GBA
- cis-3-ACPBPA
- CGP-36742 (SGS-742)
- DAVA
- Gabazine (SR-95531)
- Gaboxadol (THIP)
- I4AA
- Isonipecotic acid
- Loreclezole
- P4MPA
- P4S
- SKF-97541
- SR-95318
- SR-95813
- TPMPA
- trans-3-ACPBPA
- ZAPA
- Negative modulators: 5α-Dihydroprogesterone
- Bilobalide
- Loreclezole
- Picrotoxin (picrotin, picrotoxinin)
- Pregnanolone
- ROD-188
- THDOC
- Zinc
}}
| group2 = Metabotropic
| list2 =
| below =
- See also
- Receptor/signaling modulators
- GABAA receptor positive modulators
- GABA metabolism/transport modulators
}}
 |