Chemistry:Proligestone
 | |
| Clinical data | |
|---|---|
| Trade names | Covinan, Delvosteron |
| Other names | 14α,17α-Propylidenedioxyprogesterone; 14α,17α-Dihydroxyprogesterone cyclic acetal with propionaldehyde; 14α,17α-Dihydroxypregn-4-ene-3,20-dione cyclic acetal with propionaldehyde |
| Drug class | Progestogen; Progestin |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C24H34O4 |
| Molar mass | 386.532 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Proligestone, sold under the brand names Covinan and Delvosteron, is a progestin medication which is used in veterinary medicine.[1][2][3]
Uses
Veterinary
Proligestone is used to control estrus in dogs and cats and has also been used to treat hypersexuality in dogs and cats.[4]
Pharmacology
Pharmacodynamics
Proligestone is a progestogen, or an agonist of the progesterone receptor (PR).
Chemistry
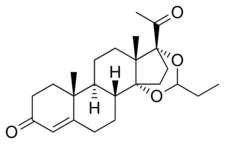
Proligestone, also known as 14α,17α-propylidenedioxyprogesterone or as 14α,17α-dihydroxyprogesterone cyclic acetal with propionaldehyde, as well as 14α,17α-propylidenedioxypregn-4-ene-3,20-dione, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[1][2] It is a C14α,17α cyclic ketal of 14α,17α-dihydroxyprogesterone.[5][6][7]
History
Proligestone was described as early as 1968 and was introduced for veterinary use in 1975.[1][8][9]
Society and culture
Generic names
Proligestone is the generic name of the drug and its INN and BAN.[1][2][3]
Brand names
Proligestone is marketed under the brand names Covinan and Delvosteron.[1][2][3]
Availability
Proligestone is or has been available for veterinary use in Argentina , Australia , Austria, Belgium, Czechoslovakia, France , Germany , Ireland, Italy, the Netherlands, New Zealand, Poland , South Africa , Switzerland , and the United Kingdom .[2][3]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 1025–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA1025.
- ↑ 2.0 2.1 2.2 2.3 2.4 Index Nominum 2000: International Drug Directory. Taylor & Francis. January 2000. pp. 882–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA882.
- ↑ 3.0 3.1 3.2 3.3 "List of Progestins". Drugs.com. https://www.drugs.com/international/proligestone.html.
- ↑ "Indications for proligestone (Delvosteron) in dogs and cats". Praktische Tierarzt 62 (12). 22 April 1981. https://eurekamag.com/research/000/908/000908843.php.
- ↑ Pharmacological and Chemical Synonyms: A Collection of Names of Drugs, Pesticides and Other Compounds Drawn from the Medical Literature of the World. Excerpta Medica. 1976. p. 376. ISBN 978-90-219-0298-2. https://books.google.com/books?id=SA-0AAAAIAAJ.
- ↑ Organic-chemical drugs and their synonyms: (an international survey). Wiley-VCH. 4 October 2001. p. 2569. ISBN 978-3-527-30247-5. https://books.google.com/books?id=1XBqAAAAMAAJ.
- ↑ "Proligestone". Medical Subject Headings: Supplementary chemical records. U.S. National Library of Medicine. https://www.ncbi.nlm.nih.gov/mesh/?term=Proligestone.
- ↑ Official Gazette of the United States Patent and Trademark Office: Trademarks. U.S. Department of Commerce, Patent and Trademark Office. 1984. p. 30. https://books.google.com/books?id=-fnQAAAAMAAJ.
- ↑ "Oestrus control in bitches with proligestone, a new progestational steroid". The Journal of Small Animal Practice 19 (9): 521–529. September 1978. doi:10.1111/j.1748-5827.1978.tb05534.x. PMID 567718.
 |

