Chemistry:Gavestinel
 | |
| Clinical data | |
|---|---|
| Other names | GV-150,526A |
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
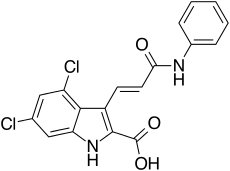
| Formula | C18H12Cl2N2O3 |
| Molar mass | 375.21 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Gavestinel (GV-150,526) was an investigational drug developed by GlaxoSmithKline for acute intracerebral hemorrhage, which in 2001 failed to show an effect in what was at the time, the largest clinical trial in stroke that had been conducted.[1][2]
Gavestinel is an NMDA antagonist, binding selectively to the glycine site on the NMDA receptor complex, rather than the glutamate site many NMDA antagonists bind to.[3][4][5]
Pharmacology and toxicology
N-methyl-D-aspartate (NMDA) receptors are amino acid receptors, overstimulation to which lead to increased intracellular Ca2+ level, and become deleterious to neural cell. In ischaemic or hypoxic conditions such as stroke, the concentration of glutamate in synaptic clefts is increased, and continuously stimulates NMDA receptors. Gavestinel was synthesized by substituting indole-2-carboxylate at the C-3 position with an unsaturated lateral side chain. It binds to NMDA receptor on the glycine site with high affinity, selectivity and a broad time window efficacy, thus gains interests in testing its efficacy in treating stroke. In pre-clinical studies, gavestinel showed no significant side effects on memory, learning, and cardiovascular system, side effects that are very common in NMDA antagonists.[6]
Clinical studies
In phase ΙΙ clinical studies to investigate safety, tolerability of gavestinel, no findings showed that it had significant side effects. The dose determined in phase ΙΙ trials was selected for further phase III trials.[7] Later, however, in two large phase III trials, gavestinel showed no efficacy in treating ischemic stroke.[8]
References
- ↑ Hauschildt, Elda (3 April 2001). "No Benefit From Early Gavestinel Therapy For Acute Stroke Patients A DGReview of :"Glycine Antagonist in Neuroprotection for Patients With Acute Stroke GAIN Americas: A Randomized Controlled Trial" Journal of the American Medical Association (JAMA)". PSL. http://www.pslclub.com/news/content.nsf/MedicalNews/8525697700573E1885256A2400487E25.
- ↑ Jeffrey, Susan (4 April 2001). "GAIN Americas trial again shows no benefit from neuroprotectant agent in stroke". Medscape News. http://www.medscape.com/viewarticle/783600.
- ↑ "Characterization of the binding of two novel glycine site antagonists to cloned NMDA receptors: evidence for two pharmacological classes of antagonists". British Journal of Pharmacology 130 (1): 65–72. May 2000. doi:10.1038/sj.bjp.0703298. PMID 10780999.
- ↑ "Why did NMDA receptor antagonists fail clinical trials for stroke and traumatic brain injury?". The Lancet. Neurology 1 (6): 383–6. October 2002. doi:10.1016/s1474-4422(02)00164-3. PMID 12849400.
- ↑ "The rise and fall of NMDA antagonists for ischemic stroke". Current Molecular Medicine 4 (2): 131–6. March 2004. doi:10.2174/1566524043479248. PMID 15032709.
- ↑ "GV150526: a neuroprotective agent.". CNS Drug Reviews 6 (2): 135–52. June 2000. doi:10.1111/j.1527-3458.2000.tb00142.x.
- ↑ "Safety and tolerability of GV150526 (a glycine site antagonist at the N-methyl-D-aspartate receptor) in patients with acute stroke". Stroke 30 (5): 986–92. May 1999. doi:10.1161/01.str.30.5.986. PMID 10229732.
- ↑ "Gavestinel does not improve outcome after acute intracerebral hemorrhage: an analysis from the GAIN International and GAIN Americas studies". Stroke 36 (5): 1006–10. May 2005. doi:10.1161/01.STR.0000163053.77982.8d. PMID 15831831.
 |

