Chemistry:Perzinfotel
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
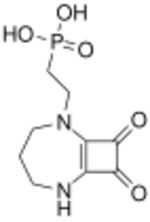
| Formula | C9H13N2O5P |
| Molar mass | 260.186 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Perzinfotel (EAA-090) is a drug which acts as a potent NMDA antagonist.[1] It has neuroprotective effects and has been investigated for the treatment of stroke,[2] but lacks analgesic effects.[3] Nevertheless, it shows a good safety profile compared to older drugs, although further development of this drug has been discontinued.[4]
Prodrugs were developed since the oral bioavailability of perzinfotel is only around 3-5%.[5]
References
- ↑ "Design and synthesis of [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)-ethyl]phosphonic acid (EAA-090), a potent N-methyl-D-aspartate antagonist, via the use of 3-cyclobutene-1,2-dione as an achiral alpha-amino acid bioisostere". Journal of Medicinal Chemistry 41 (2): 236–46. January 1998. doi:10.1021/jm970504g. PMID 9457246.
- ↑ "Characterization of two novel N-methyl-D-aspartate antagonists: EAA-090 (2-[8,9-dioxo-2,6-diazabicyclo [5.2.0]non-1(7)-en2-yl]ethylphosphonic acid) and EAB-318 (R-alpha-amino-5-chloro-1-(phosphonomethyl)-1H-benzimidazole-2-propanoic acid hydrochloride)". The Journal of Pharmacology and Experimental Therapeutics 310 (2): 563–70. August 2004. doi:10.1124/jpet.104.066092. PMID 15075380.
- ↑ "Effects of the N-methyl-D-aspartate receptor antagonist perzinfotel [EAA-090; [2-(8,9-dioxo-2,6-diazabicyclo[5.2.0]non-1(7)-en-2-yl)-ethyl]phosphonic acid] on chemically induced thermal hypersensitivity". The Journal of Pharmacology and Experimental Therapeutics 313 (3): 1379–86. June 2005. doi:10.1124/jpet.105.084467. PMID 15764736.
- ↑ "Perzinfotel". Adis International Ltd.. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800009946.
- ↑ "Prodrugs of perzinfotel with improved oral bioavailability". Journal of Medicinal Chemistry 52 (3): 771–8. February 2009. doi:10.1021/jm8011799. PMID 19146418.
 |
