Chemistry:Aptiganel
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
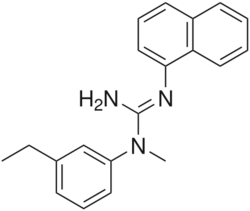
| Formula | C20H21N3 |
| Molar mass | 303.409 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Aptiganel (Cerestat; CNS-1102) is an unsuccessful drug candidate which acts as a noncompetitive NMDA antagonist, and that was under development by Cambridge Neuroscience, Inc as a treatment for stroke.[1] It has neuroprotective effects and was researched for potential use in the treatment of stroke,[2] but despite positive results in animal studies,[3] human trials showed limited efficacy,[4] as well as undesirable side effects such as sedation and hallucinations,[5][6] and clinical development was ultimately not continued.[7]
The drug's failure led to the collapse of Cambridge Neuroscience in 1998[8] and its eventual sale to CeNeS Pharmaceuticals in 2000.[9]
Other guanidine substances that the company had been bowling on was Cns-1145 & CNS1237.
Synthesis
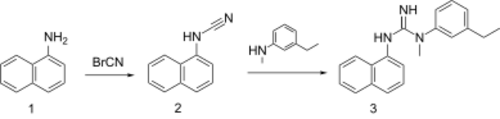
1-Naphthylamine is reacted with cyanogen bromide to give 2. Treatment of this intermediate with 3-ethyl-N-methylaniline leads to addition to the cyano group and formation of the corresponding diaryl guanidine, aptiganel, 3.
See also
- Ditolylguanidine
- CNS1237 shares predominantly most of the same structural entities.
References
- ↑ "Synthesis and structure-activity studies of N,N'-diarylguanidine derivatives. N-(1-naphthyl)-N'-(3-ethylphenyl)-N'-methylguanidine: a new, selective noncompetitive NMDA receptor antagonist". Journal of Medicinal Chemistry 37 (2): 260–7. January 1994. doi:10.1021/jm00028a009. PMID 8295213.
- ↑ "Pharmacological effects of the non-competitive NMDA antagonist CNS 1102 in normal volunteers". British Journal of Clinical Pharmacology 38 (1): 33–8. July 1994. doi:10.1111/j.1365-2125.1994.tb04318.x. PMID 7946934.
- ↑ "The N-methyl-D-aspartate antagonist CNS 1102 protects cerebral gray and white matter from ischemic injury following temporary focal ischemia in rats". Stroke 31 (7): 1709–14. July 2000. doi:10.1161/01.str.31.7.1709. PMID 10884477.
- ↑ "Aptiganel hydrochloride in acute ischemic stroke: a randomized controlled trial". JAMA 286 (21): 2673–82. December 2001. doi:10.1001/jama.286.21.2673. PMID 11730442.
- ↑ "Effects of prolonged infusions of the NMDA antagonist aptiganel hydrochloride (CNS 1102) in normal volunteers". Clinical Neuropharmacology 20 (4): 311–21. August 1997. doi:10.1097/00002826-199708000-00003. PMID 9260729.
- ↑ "Cerestat and other NMDA antagonists in ischemic stroke". Neurology 49 (5 Suppl 4): S66-9. November 1997. doi:10.1212/wnl.49.5_suppl_4.s66. PMID 9371155.
- ↑ "The rise and fall of NMDA antagonists for ischemic stroke". Current Molecular Medicine 4 (2): 131–6. March 2004. doi:10.2174/1566524043479248. PMID 15032709.
- ↑ Staff, Boston Business Journal. May 7, 1998. CNSI appoints new president, CEO
- ↑ Staff, ICIS. 23 May 2000 CeNeS to buy US neuroscience firm CNSI for $44m
- ↑ "Synthesis and structure-activity studies of N,N'-diarylguanidine derivatives. N-(1-naphthyl)-N'-(3-ethylphenyl)-N'-methylguanidine: a new, selective noncompetitive NMDA receptor antagonist". Journal of Medicinal Chemistry 37 (2): 260–7. January 1994. doi:10.1021/jm00028a009. PMID 8295213.
- ↑ Weber, Eckard & John F. W. Keana, "Tri- and tetra-substituted guanidines and their use as excitatory amino acid antagonists", WO patent 9112797, published 1991-09-05and Oregon Health Sciences University; E. Weber, J. F. W. Keana, U.S. Patent 5,262,568 (1993 to State of Oregon)
 |

