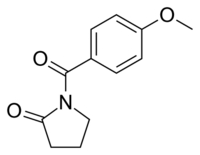
Chemistry:Aniracetam
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Ampamet, Memodrin, Pergamid |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | By mouth |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Elimination half-life | 0.5 hours[1][2] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H13NO3 |
| Molar mass | 219.240 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Aniracetam (brand names Draganon, Sarpul, Ampamet, Memodrin, Referan), also known as N-anisoyl-2-pyrrolidinone, is a racetam which is sold in Europe as a prescription drug. It is not approved by the Food and Drug Administration for use in the United States as a prescription medication or dietary supplement.[3][4] Despite the FDA's lack of approval, the drug is readily available over-the-counter in misbranded dietary supplements.[3]
Medical uses
Aniracetam has been used to treat dementia following stroke and in Alzheimer’s disease.[5] It has undergone a number of experiments in rodents; in a 1982 experiment on rats and mice it was found to have a variety of psychoactive effects, improving learning and memory that was otherwise impaired experimentally.[6] It has been identified as a nootropic drug due to these memory effects.[7] A 2001 study reported that in mice it has modest effects similar to an anxiolytic.[8]
Pharmacology
Aniracetam has been shown to positively modulate the AMPA receptor.[9]
When ingested orally aniracetam is quickly broken down via first pass hepatic metabolism. The primary metabolites of aniracetam are N-anisoyl-GABA, (70–80%), 2-Pyrrolidinone and p-anisic acid (20–30%).[2][10][11] There is some preliminary research suggesting that N-anisoyl-GABA and to a lesser degree p-ansic acid may contribute to the stimulatory effects of aniracetam in rats.[12] Further work in rats suggests that N-anisoyl-GABA may contribute more to increasing acetylcholine release than aniracetam itself.[13] For instance, a study using the forced swim test in rats found that the two metabolites 2-pyrrolidinone and N-anisoyl-GABA alone yielded similar anti-depressant effects as aniracetam itself.[12] The authors of the aforementioned study hypothesized that the metabolites work by increasing levels of dopamine and by stimulating the nicotinic acetylcholine receptors.[12]
Plasma concentrations are generally in the 5–15 μg/L range for aniracetam and 5–15 mg/L range for N-anisoyl-GABA, a pharmacologically active metabolite, during the first few hours after oral administration of the drug. These two plasma species may be measured by liquid chromatography-mass spectrometry.[14][15][16]
Synthesis
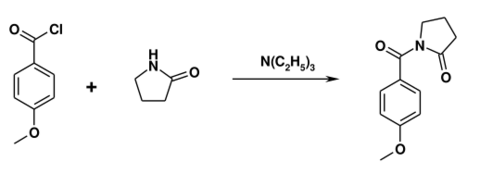
The drug was first made in the 1970s by Hoffmann-La Roche.[17][18] Synthesis can be accomplished by reacting 2-pyrrolidone with anisoyl chloride in the presence of triethylamine.[19]
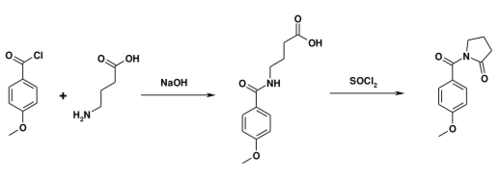
Alternatively, gamma-aminobutyric acid can react with anisoyl chloride. Ring closure can be accomplished in the presence of thionyl chloride.[19]
Legality
Europe
Aniracetam is available by prescription in Greece (brand names Memodrin and Referan) and Italy (brand name Ampamet), where it is indicated for mental function disorders.[20]
Australia
Aniracetam is a schedule 4 substance in Australia under the Poisons Standard (February 2020).[21] A schedule 4 substance is classified as "Prescription Only Medicine, or Prescription Animal Remedy – Substances, the use or supply of which should be by or on the order of persons permitted by state or territory legislation to prescribe and should be available from a pharmacist on prescription."[21]
See also
References
- ↑ "Human Pharmacokinetics of Aniracetam". Drug Investigation 5 (S1): 68–72. June 1993. doi:10.1007/BF03258428.
- ↑ 2.0 2.1 "Aniracetam. An overview of its pharmacodynamic and pharmacokinetic properties, and a review of its therapeutic potential in senile cognitive disorders". Drugs & Aging 4 (3): 257–273. March 1994. doi:10.2165/00002512-199404030-00007. PMID 8199398.
- ↑ 3.0 3.1 "Piracetam and piracetam-like drugs: from basic science to novel clinical applications to CNS disorders". Drugs 70 (3): 287–312. February 2010. doi:10.2165/11319230-000000000-00000. PMID 20166767.
- ↑ "Five Unapproved Drugs Found in Cognitive Enhancement Supplements". Neurology. Clinical Practice 11 (3): e303–e307. June 2021. doi:10.1212/CPJ.0000000000000960. PMID 34484905.
- ↑ "Aniracetam: its novel therapeutic potential in cerebral dysfunctional disorders based on recent pharmacological discoveries". CNS Drug Reviews 8 (1): 70–89. March 2002. doi:10.1111/j.1527-3458.2002.tb00216.x. PMID 12070527.
- ↑ "Effects of the novel compound aniracetam (Ro 13-5057) upon impaired learning and memory in rodents". Psychopharmacology 78 (2): 104–111. October 1982. doi:10.1007/bf00432244. PMID 6817363.
- ↑ "Aniracetam reduces glutamate receptor desensitization and slows the decay of fast excitatory synaptic currents in the hippocampus". Proceedings of the National Academy of Sciences of the United States of America 88 (23): 10936–10940. December 1991. doi:10.1073/pnas.88.23.10936. PMID 1660156. Bibcode: 1991PNAS...8810936I.
- ↑ "Anxiolytic effects of aniracetam in three different mouse models of anxiety and the underlying mechanism". European Journal of Pharmacology 420 (1): 33–43. May 2001. doi:10.1016/s0014-2999(01)01005-6. PMID 11412837.
- ↑ "Allosteric potentiation of quisqualate receptors by a nootropic drug aniracetam". The Journal of Physiology 424: 533–543. May 1990. doi:10.1113/jphysiol.1990.sp018081. PMID 1975272.
- ↑ "Clinical Trials and Studies". Schizophrenia: New Insights for the Healthcare Professional. ScholarlyEditions. 22 July 2013. pp. 152–. ISBN 978-1-4816-6196-6. https://books.google.com/books?id=zsSvvZfC8cgC&pg=PA152.
- ↑ "The Hydrolysis of Amides". Hydrolysis in Drug and Prodrug Metabolism. John Wiley & Sons. 1 August 2003. pp. 109–. ISBN 978-3-906390-25-3. https://books.google.com/books?id=U-PDqHikphYC&pg=PA109.
- ↑ 12.0 12.1 12.2 "Antidepressant-like effects of aniracetam in aged rats and its mode of action". Psychopharmacology 158 (2): 205–212. November 2001. doi:10.1007/s002130100849. PMID 11702095.
- ↑ "Group II metabotropic glutamate receptors are a common target of N-anisoyl-GABA and 1S,3R-ACPD in enhancing ACh release in the prefrontal cortex of freely moving SHRSP". Neuropharmacology 39 (5): 866–872. March 2000. doi:10.1016/s0028-3908(99)00271-3. PMID 10699452.
- ↑ "Determination of aniracetam's main metabolite, N-anisoyl-GABA, in human plasma by LC-MS/MS and its application to a pharmacokinetic study". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 897: 50–54. May 2012. doi:10.1016/j.jchromb.2012.04.007. PMID 22552003.
- ↑ "Sensitive and selective liquid chromatography-tandem mass spectrometry method for the quantification of aniracetam in human plasma". Journal of Chromatography. B, Analytical Technologies in the Biomedical and Life Sciences 858 (1–2): 129–134. October 2007. doi:10.1016/j.jchromb.2007.08.010. PMID 17826366.
- ↑ Disposition of Toxic Drugs and Chemicals in Man (10th ed.). Seal Beach, CA: Biomedical Publications. 2014. pp. 142–143. ISBN 978-0-9626523-9-4.
- ↑ Kyburz E, Aschwanden W, "p-Methoxy-benzoyl derivatives", EP patent application 44088, published 9 February 1979, assigned to Hoffmann-La Roche
- ↑ Kyburz E, Aschwanden W, "1-Benzoyl-2-pyrrolidinone derivative, processes for its preparation and medicaments containing it.", EP patent 5143, published 9 February 1979, assigned to Hoffmann-La Roche
- ↑ 19.0 19.1 Pharmaceutical substances: syntheses, patents, applications (4th ed.). Stuttgart: Thieme. 2001. ISBN 978-3-13-558404-1.
- ↑ "Classification Status of Racetams". New Zealand Medicines and Medical Devices Safety Authority. https://medsafe.govt.nz/profs/class/Agendas/agen53Racetams.pdf.
- ↑ 21.0 21.1 Poisons Standard February 2020. comlaw.gov.au
 |