Chemistry:Tulrampator
 | |
| Clinical data | |
|---|---|
| Other names | S-47445; CX-1632 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
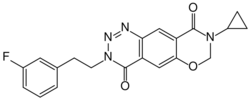
| Formula | C20H17FN4O3 |
| Molar mass | 380.379 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Tulrampator (developmental code names S-47445, CX-1632) is a positive allosteric modulator (PAM) of the AMPA receptor (AMPAR), an ionotropic glutamate receptor, which is under development by RespireRx Pharmaceuticals (formerly Cortex Pharmaceuticals) and Servier for the treatment of major depressive disorder (as an adjunct), Alzheimer's disease, dementia, and mild cognitive impairment.[1][2][3][4] Tulrampator was in phase II clinical trial for depression, but failed to show superiority over placebo.[5] There are also phase II clinical trials for Alzheimer's disease and phase I trials for dementia and mild cognitive impairment.[2]
Tulrampator is a "high-impact" AMPAR potentiator,[6] unlike "low-impact" AMPAR potentiators like CX-516 and its congener farampator (CX-691, ORG-24448), and is able to elicit more robust increases in AMPAR activation.[7] In animals, high-impact AMPAR potentiators enhance cognition and memory at low doses, but produce motor coordination disruptions, convulsions, and neurotoxicity at higher doses.[8] Tulrampator itself has been found in animals to enhance cognition and memory, to produce antidepressant-, antianhedonic-, and anxiolytic-like effects, and to have neurotrophic and neuroplasticity-promoting activities.[3][4] Moreover, it has been found to increase levels of brain-derived neurotrophic factor (BDNF) in the hippocampus and to stimulate hippocampal neurogenesis.[3][9]
The rapidly-acting antidepressant effects of the NMDA receptor antagonist ketamine appear to be mediated through indirect/downstream activation of AMPARs.[3] This is evidenced by the fact that its antidepressant-like effects in animals are blocked by the AMPAR antagonist NBQX.[3] As such, tulrampator may be a rapid-acting antidepressant similarly to ketamine but without its dissociative/hallucinogenic and certain other adverse effects (e.g., urotoxicity).[3]
See also
References
- ↑ "Tulrampatorum". WHO Drug Information 30 (4): 684. 2016-12-19. http://apps.who.int/medicinedocs/documents/s23133en/s23133en.pdf. Retrieved 2017-08-31.[|permanent dead link|dead link}}]
- ↑ 2.0 2.1 "S 47445". http://adisinsight.springer.com/drugs/800016394.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 "S 47445 Produces Antidepressant- and Anxiolytic-Like Effects through Neurogenesis Dependent and Independent Mechanisms". Front Pharmacol 8: 462. 2017. doi:10.3389/fphar.2017.00462. PMID 28769796.
- ↑ 4.0 4.1 "The AMPA receptor positive allosteric modulator S 47445 rescues in vivo CA3-CA1 long-term potentiation and structural synaptic changes in old mice". Neuropharmacology 123: 395–409. 2017. doi:10.1016/j.neuropharm.2017.06.009. PMID 28603025.
- ↑ https://clinicaltrials.servier.com/wp-content/uploads/CL2-47445-014-synopsis-report.pdf [bare URL PDF]
- ↑ "Patent US9700596 - Locally released growth factors to mediate motor recovery after stroke - Google Patents". https://www.google.com/patents/US9700596.
- ↑ "Prevention of ketamine-induced working memory impairments by AMPA potentiators in a nonhuman primate model of cognitive dysfunction". Behav. Brain Res. 212 (1): 41–8. 2010. doi:10.1016/j.bbr.2010.03.039. PMID 20347881.
- ↑ "Attenuation of ketamine-induced impairment in verbal learning and memory in healthy volunteers by the AMPA receptor potentiator PF-04958242". Mol. Psychiatry 22 (11): 1633–1640. 2017. doi:10.1038/mp.2017.6. PMID 28242871.
- ↑ "Upregulation of neurotrophins by S 47445, a novel positive allosteric modulator of AMPA receptors in aged rats". Pharmacol. Res. 121: 59–69. 2017. doi:10.1016/j.phrs.2017.04.019. PMID 28442348. https://air.unimi.it/bitstream/2434/548234/4/42.CalabresePR2017.pdf.
External links
 |
