Chemistry:Tezampanel
 | |
| Clinical data | |
|---|---|
| Routes of administration | IV |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
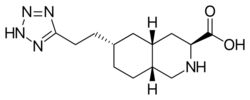
| Formula | C13H21N5O2 |
| Molar mass | 279.344 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tezampanel (INN, USAN) (code names LY-293,558, NGX-424) is a drug originally developed by Eli Lilly[1] which acts as a competitive antagonist of the AMPA and kainate subtypes of the ionotropic glutamate receptor family,[2][3] with selectivity for the GluR5 subtype of the kainate receptor.[4][5] It has neuroprotective[6] and anticonvulsant properties,[7] the former of which may, at least in part, occur via blockade of calcium uptake into neurons.[8]
Tezampanel has a range of effects which may be useful for medicinal purposes, as well as its applications in scientific research. It suppresses both the withdrawal symptoms from morphine and other opioids,[9][10][11] and the development of tolerance,[12] as well as having antihyperalgesic[13] and analgesic effects in its own right.[14][15][16][17][18] It also has anxiolytic effects in animal studies and has been suggested as a candidate for the treatment of anxiety in humans.[19]
References
- ↑ "LY-293558. Eli Lilly & Co". Current Opinion in Investigational Drugs 2 (9): 1273–8. September 2001. PMID 11717815.
- ↑ "(3SR,4aRS,6RS,8aRS)-6-[2-(1H-tetrazol-5-yl)ethyl]decahydroisoquinoline-3 - carboxylic acid: a structurally novel, systemically active, competitive AMPA receptor antagonist". Journal of Medicinal Chemistry 36 (14): 2046–8. July 1993. doi:10.1021/jm00066a016. PMID 8393116.
- ↑ "In vitro and in vivo antagonism of AMPA receptor activation by (3S, 4aR, 6R, 8aR)-6-[2-(1(2)H-tetrazole-5-yl) ethyl] decahydroisoquinoline-3-carboxylic acid". Neuropharmacology 34 (9): 1159–68. September 1995. doi:10.1016/0028-3908(95)00099-r. PMID 8532186.
- ↑ "Pharmacological discrimination of GluR5 and GluR6 kainate receptor subtypes by (3S,4aR,6R,8aR)-6-[2-(1(2)H-tetrazole-5-yl)ethyl]decahyd roisdoquinoline-3 carboxylic-acid". Molecular Pharmacology 49 (4): 581–5. April 1996. PMID 8609884.
- ↑ "GluR5 kainate receptor mediated synaptic transmission in rat basolateral amygdala in vitro". Neuropharmacology 37 (10–11): 1279–86. 1998. doi:10.1016/s0028-3908(98)00109-9. PMID 9849665.
- ↑ "Neuroprotective effect of the AMPA receptor antagonist LY-293558 in focal cerebral ischemia in the cat". Journal of Cerebral Blood Flow and Metabolism 14 (3): 466–71. May 1994. doi:10.1038/jcbfm.1994.57. PMID 8163588.
- ↑ "Role of AMPA and GluR5 kainate receptors in the development and expression of amygdala kindling in the mouse". Neuropharmacology 40 (1): 28–35. 2001. doi:10.1016/s0028-3908(00)00112-x. PMID 11077068.
- ↑ "Effects of decahydroisoquinoline-3-carboxylic acid monohydrate, a novel AMPA receptor antagonist, on glutamate-induced CA2+ responses and neurotoxicity in rat cortical and cerebellar granule neurons". Biochemical Pharmacology 50 (11): 1761–74. November 1995. doi:10.1016/0006-2952(95)02032-2. PMID 8615854.
- ↑ "A selective AMPA antagonist, LY293558, suppresses morphine withdrawal-induced activation of locus coeruleus neurons and behavioral signs of morphine withdrawal". Neuropsychopharmacology 15 (5): 497–505. November 1996. doi:10.1016/S0893-133X(96)00094-2. PMID 8914123.
- ↑ "The competitive alpha-amino-3-hydroxy-5-methylisoxazole-4-propionate receptor antagonist LY293558 attenuates and reverses analgesic tolerance to morphine but not to delta or kappa opioids". The Journal of Pharmacology and Experimental Therapeutics 283 (3): 1249–55. December 1997. PMID 9400000.
- ↑ "The effects of LY293558, an AMPA receptor antagonist, on acute and chronic morphine dependence". Brain Research 778 (1): 120–6. December 1997. doi:10.1016/s0006-8993(97)00985-2. PMID 9462883.
- ↑ "AMPA antagonist LY293558 blocks the development, without blocking the expression, of behavioral sensitization to morphine". Synapse 31 (4): 256–62. March 1999. doi:10.1002/(SICI)1098-2396(19990315)31:4<256::AID-SYN3>3.0.CO;2-E. PMID 10051106.
- ↑ "AMPA/kainate antagonist LY293558 reduces capsaicin-evoked hyperalgesia but not pain in normal skin in humans". Anesthesiology 89 (5): 1060–7. November 1998. doi:10.1097/00000542-199811000-00005. PMID 9821993.
- ↑ "Effects of the 2-amino-3-hydroxy-5-methyl-4-isoxazole-proprionic acid/kainate antagonist LY293558 on spontaneous and evoked postoperative pain". Clinical Pharmacology and Therapeutics 68 (3): 320–7. September 2000. doi:10.1067/mcp.2000.108677. PMID 11014414.
- ↑ "Effect of intrathecal non-NMDA EAA receptor antagonist LY293558 in rats: a new class of drugs for spinal anesthesia". Anesthesiology 97 (1): 177–82. July 2002. doi:10.1097/00000542-200207000-00025. PMID 12131120.
- ↑ "LY293558, a novel AMPA/GluR5 antagonist, is efficacious and well-tolerated in acute migraine". Cephalalgia 24 (7): 596–602. July 2004. doi:10.1111/j.1468-2982.2004.00723.x. PMID 15196302.
- ↑ "The effect of the AMPA/kainate receptor antagonist LY293558 in a rat model of postoperative pain". The Journal of Pain 7 (10): 768–77. October 2006. doi:10.1016/j.jpain.2006.03.010. PMID 17018337.
- ↑ "Epidural tezampanel, an AMPA/kainate receptor antagonist, produces postoperative analgesia in rats". Anesthesia and Analgesia 105 (4): 1152–9, table of contents. October 2007. doi:10.1213/01.ane.0000281435.58012.e3. PMID 17898404.
- ↑ "In vitro and in vivo studies in rats with LY293558 suggest AMPA/kainate receptor blockade as a novel potential mechanism for the therapeutic treatment of anxiety disorders". Psychopharmacology 185 (2): 240–7. April 2006. doi:10.1007/s00213-005-0292-0. PMID 16470401.
 |

