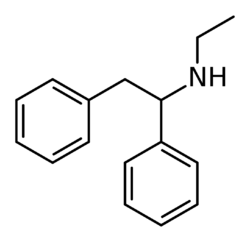
Chemistry:Ephenidine
 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C16H19N |
| Molar mass | 225.335 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Ephenidine (also known as NEDPA and EPE) is a dissociative anesthetic that has been sold online as a designer drug.[1][2] It is illegal in some countries as a structural isomer of the banned opioid drug lefetamine, but has been sold in countries where it is not yet banned.[3][4]
Pharmacology
Pharmacodynamics
Ephenidine and related diarylethylamines have been studied in vitro as treatments for neurotoxic injuries, and are antagonists of the NMDA receptor (Ki = 66.4 nM for ephenidine).[5][6][7][8][9] Ephenidine also possesses weaker affinity for dopamine and norepinephrine transporters (379 nM and 841 nM, respectively) as well as σ1R (629 nM) and σ2R (722 nM) binding sites.[10]
Pharmacokinetics
Metabolism
Ephenidine's metabolic pathway consists of N-oxidation, N-dealkylation, mono- and bis-hydroxylation of the benzyl ring, and hydroxylation of the phenyl ring only after N-dealkylation. The dihydroxy metabolites were conjugated by methylation of one hydroxy group, and hydroxy metabolites by glucuronidation or sulfation.[3][11]
Chemistry
Ephenidine reacts with reagent testing kits to give a semi-unique array of colors which can be used to aid its identification.
| Reagent | Reaction color |
|---|---|
| Marquis | Orange > Brown |
| Mandelin | Green |
| Liebermann | Deep red > Brown (fast) |
| Froehde | Light Yellow |
Society and culture
Sweden's public health agency suggested that ephenidine be classified as a hazardous substance on 1 June, 2015. Due to that suggestion, ephenidine became a scheduled substance, in Sweden, as of 18 August, 2015.[13]
In 2016, Canada added MT-45 and "its salts, derivatives, isomers and analogues" to the Schedule I controlled substance list, and explicitly included ephenidine.[14] Possession without legal authority can result in maximum 7 years imprisonment.
See also
- AD-1211
- βk-Ephenidine
- Diphenidine
- Fluorolintane
- Lanicemine
- Methoxphenidine (MXP)
- MT-45
- NPDPA
- Remacemide
- UWA-001
References
- ↑ "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis 6 (7–8): 614–32. July–August 2014. doi:10.1002/dta.1620. PMID 24678061.
- ↑ "Michaelis-Menten kinetic analysis of drugs of abuse to estimate their affinity to human P-glycoprotein". Toxicology Letters 217 (2): 137–42. February 2013. doi:10.1016/j.toxlet.2012.12.012. PMID 23273999.
- ↑ 3.0 3.1 "Lefetamine-derived designer drugs N-ethyl-1,2-diphenylethylamine (NEDPA) and N-iso-propyl-1,2-diphenylethylamine (NPDPA): metabolism and detectability in rat urine using GC-MS, LC-MSn and LC-HR-MS/MS". Drug Testing and Analysis 6 (10): 1038–48. October 2014. doi:10.1002/dta.1621. PMID 24591097.
- ↑ "Toxicokinetics of lefetamine and derived diphenylethylamine designer drugs-Contribution of human cytochrome P450 isozymes to their main phase I metabolic steps". Toxicology Letters 238 (3): 39–44. November 2015. doi:10.1016/j.toxlet.2015.08.012. PMID 26276083.
- ↑ "Patent EP 0346791 - 1,2-diarylethylamines for treatment of neurotoxic injury". G.D. Searle, LLC. 6 April 1994. https://www.surechembl.org/document/EP-0346791-B1/.
- ↑ "NMDA receptor affinities of 1,2-diphenylethylamine and 1-(1,2-diphenylethyl)piperidine enantiomers and of related compounds". Bioorganic & Medicinal Chemistry 17 (9): 3456–62. May 2009. doi:10.1016/j.bmc.2009.03.025. PMID 19345586.
- ↑ "Preparation and characterization of the 'research chemical' diphenidine, its pyrrolidine analogue, and their 2,2-diphenylethyl isomers". Drug Testing and Analysis 7 (5): 358–67. May 2015. doi:10.1002/dta.1689. PMID 25044512. http://researchonline.ljmu.ac.uk/id/eprint/3408/1/DTA-14-0117.R1.pdf. Retrieved 2021-05-31.
- ↑ "Structural and conformational aspects of the binding of aryl-alkyl amines to the phencyclidine binding site". NIDA Research Monograph 95: 51–6. 1989. PMID 2561843. http://archives.drugabuse.gov/pdf/monographs/95.pdf. Retrieved 2016-08-11.
- ↑ "Analgesics; n-alkylated-1,2-diphenylethylamines prepared by the Leuckart reaction". Journal of the American Chemical Society 68 (11): 2174–2175. November 1946. doi:10.1021/ja01215a018. PMID 21002222.
- ↑ "Ephenidine: A new psychoactive agent with ketamine-like NMDA receptor antagonist properties". Neuropharmacology 112 (Pt A): 144–149. January 2017. doi:10.1016/j.neuropharm.2016.08.004. PMID 27520396.
- ↑ "Lefetamine, a controlled drug and pharmaceutical lead of new designer drugs: synthesis, metabolism, and detectability in urine and human liver preparations using GC-MS, LC-MS(n), and LC-high resolution-MS/MS". Analytical and Bioanalytical Chemistry 407 (6): 1545–57. February 2015. doi:10.1007/s00216-014-8414-3. PMID 25577353.
- ↑ "Ephenidine reaction results with various reagent tests". Reagent Tests UK. 17 January 2016. https://www.reagent-tests.uk/blog/ephenidine-reaction-results-with-various-reagent-tests/.
- ↑ "23 nya ämnen kan klassas som narkotika eller hälsofarlig vara" (in sv). Folkhälsomyndigheten. 1 June 2015. https://www.folkhalsomyndigheten.se/nyheter-och-press/nyhetsarkiv/2015/juni/23-nya-amnen-kan-klassas-som-narkotika-eller-halsofarlig-vara.
- ↑ "Regulations Amending the Food and Drug Regulations (Parts G and J — Lefetamine, AH-7921, MT-45 and W-18)". Canada Gazette 150 (11). 1 June 2016. http://www.gazette.gc.ca/rp-pr/p2/2016/2016-06-01/html/sor-dors106-eng.php. Retrieved 2016-11-17.
 |

