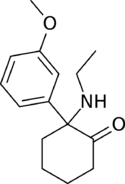
Chemistry:Methoxetamine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | MXE; 3-MeO-2'-oxo-PCE |
| Addiction liability | High[1] |
| Drug class | NMDA receptor antagonists; Dissociative hallucinogens; General anesthetics |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 3–6 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C15H21NO2 |
| Molar mass | 247.338 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Methoxetamine, abbreviated as MXE, is a dissociative hallucinogen that has been sold as a designer drug.[4][5] It differs from many dissociatives such as ketamine and phencyclidine (PCP) that were developed as pharmaceutical drugs for use as general anesthetics in that it was designed specifically to increase the antidepressant effects of ketamine.[5][6]
MXE is an arylcyclohexylamine.[7] It acts mainly as an NMDA receptor antagonist, similarly to other arylcyclohexylamines like ketamine and PCP.[7]
Recreational use
Effects

MXE is reported to have a similar effect to ketamine.[1] It was often believed to possess opioid properties due to its structural similarity to 3-HO-PCP,[5] but this assumption is not supported by data, which shows insignificant affinity for the μ-opioid receptor by the compound.[7] Recreational use of MXE has been associated with hospitalizations from high and/or combined consumption in the US and UK.[8][9][10] Acute reversible cerebellar toxicity has been documented in three cases of hospital admission due to MXE overdose, lasting for between one and four days after exposure.[9]
MXE was designed in part in an attempt to avoid the urotoxicity associated with ketamine abuse; it was thought the compound's increased potency and reduced dose would limit the accumulation of urotoxic metabolites in the bladder.[5][6] Like ketamine, MXE has been found to produce bladder inflammation and fibrosis after high dose chronic administration in mice, although the dosages used were quite large.[11] Reports of urotoxicity in humans have yet to appear in the medical literature.[5]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Action | Species | Ref |
|---|---|---|---|---|
| NMDA (PCP) |
259 | Antagonist | Human | [7] |
| MOR | >10,000 | ND | Human | [7] |
| DOR | >10,000 | ND | Human | [7] |
| KOR | >10,000 | ND | Human | [7] |
| NOP | >10,000 | ND | Human | [7] |
| σ1 | >10,000 | ND | Guinea pig | [7] |
| σ2 | >10,000 | ND | Rat | [7] |
| D2 | >10,000 | ND | Human | [7] |
| 5-HT2A | >10,000 | ND | Human | [7] |
| NET | >10,000 20,000 (IC50) |
Inhibitor | Human | [7] [12] |
| DAT | >10,000 33,000 (IC50) |
Inhibitor | Human | [7] [12] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
MXE acts mainly as a selective and high-affinity NMDA receptor antagonist, specifically of the dizocilpine (MK-801) site (Ki = 257 nM).[7][13] It produces ketamine-like effects.[13][14] In addition to antagonism of the NMDA receptor, MXE has been found to act as a serotonin reuptake inhibitor (Ki = 479 nM; IC50 = 2,400 nM).[7][12] Conversely, it shows little or no effect on the reuptake of dopamine and norepinephrine (Ki and IC50 > 10,000 nM).[7][12] Nonetheless, MXE has been found to activate dopaminergic neurotransmission, including in the mesolimbic reward pathway.[13] This is a characteristic that it shares with other NMDA receptor antagonists, including ketamine, PCP, and dizocilpine (MK-801).[13] Animal studies suggest MXE may have rapidly-acting antidepressant effects similar to those of ketamine.[13][15] A study that assessed binding of MXE at 56 sites including neurotransmitter receptors and transporters found that MXE had Ki values of >10,000 nM for all sites except the dizocilpine site of the NMDA receptor and the serotonin transporter (SERT).[7]
Pharmacokinetics
MXE has a longer duration of action than that of ketamine.[16]
Chemistry

MXE is an arylcyclohexylamine and a derivative of eticyclidine (PCE). It can also be thought of as the β-Keto-derivative of 3-methoxyeticyclidine (3-MeO-PCE), or the N-ethyl homologue of methoxmetamine (MXM) and methoxpropamine (MXPr). It is closely related structurally to ketamine, and more distantly to PCP.
MXE hydrochloride is soluble in ethanol up to 10 mg/ml at 25 °C.[17]
Detection in body fluids
A forensic standard of MXE is available, and the compound has been posted on the Forendex website of potential drugs of abuse.[18]
History
The qualitative effects of MXE were first described online in May 2010 and the compound became commercially available on a small scale in September 2010.[4][5] By November the use and sale of the MXE had increased enough for it to be formally identified by the European Monitoring Centre for Drugs and Drug Addiction. By July 2011, the EMCDDA had identified 58 websites selling the compound at a cost of 145–195 euros for 10 grams.[19]
Society and culture

Media coverage
Mixmag reported in January 2012, that people in the dance music and clubbing community have given MXE the slang name 'roflcoptr'.[20] Vice commented that it was likely that the phrase will only be used by "the same politicians, parents and journalists" who called mephedrone 'meow meow'.[21] After being called mexxy in UK Home Office press releases, the media adopted the name.[10][22]
A literature review was published in March 2012 which looked at scientific literature and information on the web. It concluded that "the online availability of information on novel psychoactive drugs, such as MXE, may constitute a pressing public health challenge. Better international collaboration levels and novel forms of intervention are necessary to tackle this fast-growing phenomenon."[23]
Legal status
Brazil
MXE became classified as a narcotic in Brazil in February 2014.[24]
Canada
As of January 2010 MXE is a controlled substance in Canada.[25]
China
As of October 2015 MXE is a controlled substance in China.[26]
European Union
On 16 June 2014, the European Commission proposed that MXE be banned across the European Union, subjecting those in violation to criminal sanctions. This is following the procedure for risk-assessment and control of new psychoactive substances set up by the council: Decision 2005/387/JHA.[27]
Israel
MXE became classified as an illegal narcotic in Israel in May 2012.[28][29]
Japan
MXE became a controlled substance in Japan from 1 July 2012, by amendment to the Pharmaceutical Affairs Law.[30][31]
Poland
MXE is a controlled substance (group II-P) making it illegal to produce, sell or possess in The Republic of Poland as of 1 July 2015.[32]
Russia
MXE has been a controlled substance in Russia since October 2011.[33]
Sweden
MXE became classified as a narcotic in Sweden in late February 2012.[34]
Switzerland
MXE has been illegal in Switzerland since December 2011.[35]
United Kingdom
Prior to March 2012, MXE was not controlled by the UK's Misuse of Drugs Act.[36] In March 2012, the Home Office referred MXE to the Advisory Council on the Misuse of Drugs for possible temporary controlling under the powers given in the Police Reform and Social Responsibility Act 2011.[37][38] The ACMD gave their advice on 23 March, with the chair commenting that "the evidence shows that the use of methoxetamine can cause harm to users and the ACMD advises that it should be subject to a temporary class drug order."[39] In April 2012, MXE was placed under temporary class drug control, which prohibited its import and sale for 12 months.[40]
- Theresa May commented in her reply to the ACMD that "the next step in this process is for the ACMD to undertake a full assessment of MXE for consideration for its permanent control under the 1971 Act." She goes on to say that she hopes the ACMD will do this as a part of the review of ketamine, "including its analogues" and that this review will be completed "within the 12 months from the making of the current order".[41]
- On 18 October 2012 the ACMD released a report about MXE, saying that the "harms of methoxetamine are commensurate with Class B of the Misuse of Drugs Act (1971)", despite the fact that the act does not classify drugs based on harm. The report went on to suggest that all analogues of MXE should also become class B drugs and suggested a catch-all clause covering both existing and unresearched arylcyclohexamines.
- MXE ceased to be covered by the temporary prohibition on 26 February 2013, when it became classified as a Class B drug.[42]
United Nations
MXE was made a schedule II drug in November 2016.[43]
United States
On June 6, 2022, the U.S. Drug Enforcement Administration published a final rule placing MXE in Schedule I of the Controlled Substances Act.[44]
- Alabama
- MXE is a Schedule I controlled substance in the state of Alabama making it illegal to buy, sell, or possess in Alabama.[45]
- Florida
- MXE is a Schedule I controlled substance in the state of Florida making it illegal to buy, sell, or possess in Florida.[46]
- Utah
- MXE is a controlled substance in the state of Utah making it illegal to buy, sell, or possess in Utah.[47]
References
- ↑ 1.0 1.1 "Methoxetamine (MXE)--a phenomenological study of experiences induced by a "legal high" from the internet". Journal of Psychoactive Drugs 45 (3): 276–286. 2013. doi:10.1080/02791072.2013.803647. PMID 24175493.
- ↑ "Federal Register". FederalRegister.gov. https://www.federalregister.gov/documents/2022/06/06/2022-11933/schedules-of-controlled-substances-placement-of-methoxetamine-mxe-in-schedule-i.
- ↑ "Substance Details Methoxetamine". https://www.unodc.org/LSS/Substance/Details/a7c33ea4-a751-4586-aeb5-46b567236bc3.
- ↑ 4.0 4.1 4.2 EMCDDA Annual Report 2010 (Report). European Monitoring Centre for Drugs and Drug Addiction. 2010. http://www.emcdda.europa.eu/attachements.cfm/att_132857_EN_EMCDDA-Europol%20Annual%20Report%202010A.pdf. Retrieved 2012-01-23.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 "From PCP to MXE: a comprehensive review of the non-medical use of dissociative drugs". Drug Testing and Analysis 6 (7–8): 614–632. 2014. doi:10.1002/dta.1620. PMID 24678061.
- ↑ 6.0 6.1 "Interview with a ketamine chemist: or to be more precise, an arylcyclohexylamine chemist". Vice Magazine. 2011-02-11. https://www.vice.com/read/interview-with-ketamine-chemist-704-v18n2.
- ↑ 7.00 7.01 7.02 7.03 7.04 7.05 7.06 7.07 7.08 7.09 7.10 7.11 7.12 7.13 7.14 7.15 7.16 7.17 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid23527166 - ↑ "Acute toxicity associated with the recreational use of the ketamine derivative methoxetamine". European Journal of Clinical Pharmacology 68 (5): 853–856. May 2012. doi:10.1007/s00228-011-1199-9. PMID 22205276.
- ↑ 9.0 9.1 "Methoxetamine associated reversible cerebellar toxicity: three cases with analytical confirmation". Clinical Toxicology 50 (5): 438–440. June 2012. doi:10.3109/15563650.2012.683437. PMID 22578175.
- ↑ 10.0 10.1 "Pair hospitalised after taking designer drug mexxy". BBC News. 7 April 2014. https://www.bbc.com/news/uk-wales-north-west-wales-26928186.
- ↑ "Three months of methoxetamine administration is associated with significant bladder and renal toxicity in mice". Clinical Toxicology 52 (3): 176–180. March 2014. doi:10.3109/15563650.2014.892605. PMID 24580056.
- ↑ 12.0 12.1 12.2 12.3 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid28454981 - ↑ 13.0 13.1 13.2 13.3 13.4 "Methoxetamine, a novel psychoactive substance with serious adverse pharmacological effects: a review of case reports and preclinical findings". Behavioural Pharmacology 27 (6): 489–496. September 2016. doi:10.1097/FBP.0000000000000241. PMID 27128862.
- ↑ "Ketamine-like effects after recreational use of methoxetamine". Annals of Emergency Medicine 60 (1): 97–99. July 2012. doi:10.1016/j.annemergmed.2011.11.018. PMID 22237166.
- ↑ "Methoxetamine produces rapid and sustained antidepressant effects probably via glutamatergic and serotonergic mechanisms". Neuropharmacology 126: 121–127. November 2017. doi:10.1016/j.neuropharm.2017.08.038. PMID 28867363.
- ↑ "Detailed pharmacological evaluation of methoxetamine (MXE), a novel psychoactive ketamine analogue-Behavioural, pharmacokinetic and metabolic studies in the Wistar rat". Brain Research Bulletin 126 (Pt 1): 102–110. September 2016. doi:10.1016/j.brainresbull.2016.05.002. PMID 27155360.
- ↑ "Methoxamine (hydrochloride) Safety Data Sheet". Caymen Chemicals. https://www.caymanchem.com/msdss/11139m.pdf.
- ↑ Southern Association of Forensic Scientists "Methoxetamine". http://forendex.safs1966.org/index.php/detail/index/602.
- ↑ Online sales of new psychoactive substances/'legalhighs': Summary of results from the 2011 multilingual snapshots (Report). European Monitoring Centre for Drugs and Drug Addiction. 2011-11-15. http://www.emcdda.europa.eu/attachements.cfm/att_143801_EN_SnapshotSummary.pdf. Retrieved 2012-01-23.
- ↑ "Methoxetamine is a new chemical analogue of ketamine. It's legal, it's cheap and it's trippy as hell - but is it safe?". Mixmag (London, UK) (249): p. 60. 2012-01-18.
- ↑ "We Interviewed the Inventor of Roflcoptr, the New Drug Britain's Panicking About". Vice Magazine. 2011-02-11. https://www.vice.com/en_uk/read/we-interviewed-the-man-who-invented-roflecoptr.
- ↑ "Legal high drug 'mexxy' banned under new government powers". The Guardian (London). 28 March 2012. https://www.theguardian.com/society/2012/mar/28/legal-high-drug-methoxetamine-banned.
- ↑ "Phenomenon of new drugs on the Internet: the case of ketamine derivative methoxetamine". Human Psychopharmacology 27 (2): 145–149. March 2012. doi:10.1002/hup.1242. PMID 22389078.
- ↑ "Anvisa inclui 21 substâncias em lista de drogas proibidas" (in pt). http://portal.anvisa.gov.br/wps/content/anvisa+portal/anvisa/sala+de+imprensa/assunto+de+interesse/noticias/anvisa+inclui+21+substancias+em+lista+de+drogas+proibidas.
- ↑ "Status Decision Of Controlled And Non-Controlled Substance(s)". 2011-01-31. http://isomerdesign.com/Cdsa/HC/StatusDecisions/A-2013-00235%20-%20PDFs/C-Methoxetamine-2011-01-31.pdf.
- ↑ "关于印发《非药用类麻醉药品和精神药品列管办法》的通知" (in zh). China Food and Drug Administration. 27 September 2015. http://www.sfda.gov.cn/WS01/CL0056/130753.html.
- ↑ "Legal highs: European Commission takes strong action with EU-wide ban on four new substances". European Commission. 16 June 2014. http://europa.eu/rapid/midday-express-16-06-2014.htm.
- ↑ "פקודת הסמים המסוכנים [נוסח חדש, תשל"ג-1973"] (in he). https://he.wikisource.org/wiki/%D7%A4%D7%A7%D7%95%D7%93%D7%AA_%D7%94%D7%A1%D7%9E%D7%99%D7%9D_%D7%94%D7%9E%D7%A1%D7%95%D7%9B%D7%A0%D7%99%D7%9D#.D7.AA.D7.95.D7.A1.D7.A4.D7.AA_1_.D7.97.D7.9C.D7.A7_.D7.90_.D7.A1.D7.99.D7.9E.D7.9F_.D7.91.
- ↑ "הודעת הסמים המסוכנים (שינוי התוספת הראשונה לפקודה) (מס" 2), התשע"ב-2012" (in he). https://oknesset.org/committee/meeting/6050/.
- ↑ "Error: no
|title=specified when using {{Cite web}}" (in ja). http://www.fukushihoken.metro.tokyo.jp/kenkou/iyaku/d_taisaku/yakuji/.[yes|permanent dead link|dead link}}] - ↑ "Methoxetamine Critical Report Review". https://www.who.int/medicines/access/controlled-substances/5.9_Methoxetamine_CRev.pdf.
- ↑ "Ustawa z dnia 29 lipca 2005 r. o przeciwdziałaniu narkomanii". http://isap.sejm.gov.pl/Download?id=WDU20051791485&type=3.
- ↑ "Resolution of the Government of the Russian Federation on October 6, 2011 N 822" (in ru). http://www.rg.ru/2011/10/19/narko-dok.html.
- ↑ "Narkotika-klassning av sex nya substanser" (in sv). http://www.fhi.se/Aktuellt/Nyheter/Narkotika-klassning-av-sex-nya-substanser/.
- ↑ "Ordinance on the lists of narcotic drugs, psychotropic substances, precursors and auxiliary chemicals" (in de). http://www.admin.ch/dokumentation/gesetz/00068/index.html?lang=de&download=NHzLpZeg7t,lnp6I0NTU042l2Z6ln1acy4Zn4Z2qZpnO2Yuq2Z6gpJCDeHx2gWym162epYbg2c_JjKbNoKSn6A--.
- ↑ "Health alert over drug sold as 'safe ketamine'". The Independent (London). 2012-02-13. https://www.independent.co.uk/life-style/health-and-families/health-news/health-alert-over-drug-sold-as-safe-ketamine-6804824.html.
- ↑ "Safe ketamine' referred to drug experts". 2012-03-06. https://www.gov.uk/government/news/safe-ketamine-referred-to-drug-experts.
- ↑ "Bid to ban 'safe' drug Methoxetamine after deaths". The Independent (London). 2012-03-06. https://www.independent.co.uk/life-style/health-and-families/health-news/bid-to-ban-safe-drug-methoxetamine-after-deaths-7542175.html.
- ↑ "Statement of evidence - Methoxetamine". 2012-02-27. https://www.gov.uk/government/publications/statement-of-evidence-methoxetamine.
- ↑ "Methoxetamine". UK Home Office. http://www.homeoffice.gov.uk/drugs/drug-law/temporary-class-drug-orders/methoxetamine/.
- ↑ "Home Secretary's response to the ACMD's advice on methoxetamine". 2012-03-27. https://www.gov.uk/government/publications/home-secretarys-response-to-the-acmds-advice-on-methoxetamine.
- ↑ "Home Office circular 004-2013". 2013-02-20. https://www.gov.uk/government/publications/change-to-the-misuse-of-drugs-act-1971.
- ↑ "UNODC: Commission on Narcotic Drugs decision on international control of PMMA, α-PVP, 4,4'-DMAR, MXE and Phenazepam enters into force". 13 November 2016. https://www.unodc.org/LSS/Announcement/Details/6dd8eae4-7b30-4ae1-889e-f8a03d62df18.
- ↑ "Schedules of Controlled Substances: Placement of Methoxetamine (MXE) in Schedule I". Federal Register. 6 June 2022. https://www.federalregister.gov/documents/2022/06/06/2022-11933/schedules-of-controlled-substances-placement-of-methoxetamine-mxe-in-schedule-i.
- ↑ "Chapter 420-7-2 Controlled Substances". Alabamma State Board of Health. http://www.alabamaadministrativecode.state.al.us/docs/hlth/420-7-2.pdf.
- ↑ "Statutes & Constitution :View Statutes : Online Sunshine". http://leg.state.fl.us/statutes/index.cfm?App_mode=Display_Statute&URL=0800-0899/0893/0893.html.
- ↑ Utah Code 58-37-4.2. Listed controlled substances.
External links
 |
