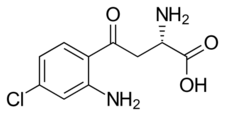
Chemistry:4-Chlorokynurenine
 | |
 | |
| Clinical data | |
|---|---|
| Other names | 4-Cl-KYN; AV-101; 3-(4-Chloroanthraniloyl)-DL-alanine |
| Pregnancy category |
|
| Routes of administration | By mouth |
| Drug class | NMDA receptor antagonist |
| ATC code |
|
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | 39–84% (rodents); ≥ 31% (humans)[citation needed] |
| Elimination half-life | 2–3 hours[citation needed] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C10H11ClN2O3 |
| Molar mass | 242.66 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
L-4-Chlorokynurenine (4-Cl-KYN; developmental code name AV-101) is an orally active small molecule prodrug of 7-chlorokynurenic acid, a NMDA receptor antagonist. It was investigated as a potential rapid-acting antidepressant.
AV-101 was discovered at Marion Merrell Dow and its biological activity was explored at University of Maryland. It underwent initial development at Artemis Neuroscience which was acquired by VistaGen in 2003. A phase II clinical trial failed to show any effect over placebo in alleviating treatment-resistant depression.[1]
Pharmacology

4-Chlorokynurenine penetrates the blood–brain barrier via the large neutral amino acid transporter 1.[3] In the central nervous system it is converted to 7-chlorokynurenic acid by kynurenine aminotransferase in astrocytes.[4]
Most of its therapeutic potential is believed to occur via 7-chlorokynurenic acid which inhibits the glycine co-agonist site of NMDA receptors.[4]
Another metabolite, 4-chloro-3-hydroxy-anthranilic acid, inhibits the enzyme 3-hydroxyanthranilate oxidase, which provides a rationale for further testing in neurodegenerative diseases.[4]
Chemistry
4-Chlorokynurenine is prodrug of 7-chlorokynurenic acid (7-Cl-KYNA), which in turn is a halogenated derivative of L-kynurenine.[4]
History
Artemis Neuroscience was formed to develop work done by University of Maryland professor Robert Schwartz in collaboration with scientists at Marion Merrell Dow (which became part of Sanofi by way of Aventis); this work included AV-101.[5][6][7]
VistaGen acquired AV-101 when it acquired Artemis in 2003.[8]
VistaGen filed an Investigational New Drug application with the FDA for use of AV-101 in neuropathic pain in 2013.[4]
In 2013, other NMDA receptor antagonists in clinical trials for depression included lanicemine, esketamine, and rapastinel, with lanicemine being the most advanced.[9]
Research
By 2013, AV-101 had successfully gone through two Phase I clinical trials.[4] In 2016, a Phase II clinical trial was initiated to assess AV-101 in treatment-resistant major depression.[10] The trial found no difference in treatment effects between AV-101 and placebo.[1][11]
Preclinical studies in animal models suggested efficacy in treating neuropathic pain.[12] AV-101 showed efficacy in an animal model of Huntington's disease[4] and rapid-acting antidepressant effects similar to ketamine in behavioral models of depression in rodents.[10]
See also
References
- ↑ 1.0 1.1 "A Randomized Trial of the N-Methyl-d-Aspartate Receptor Glycine Site Antagonist Prodrug 4-Chlorokynurenine in Treatment-Resistant Depression". The International Journal of Neuropsychopharmacology 23 (7): 417–425. July 2020. doi:10.1093/ijnp/pyaa025. PMID 32236521.
- ↑ "Molecular determinants of agonist discrimination by NMDA receptor subunits: analysis of the glutamate binding site on the NR2B subunit". Neuron 18 (3): 493–503. March 1997. doi:10.1016/S0896-6273(00)81249-0. PMID 9115742.
- ↑ "11. Prodrug Approaches for Central Nervous System Delivery". Prodrugs and Targeted Delivery: Towards Better ADME Properties Volume 47 of Methods and Principles in Medicinal Chemistry. John Wiley & Sons. 2011. p. 259. ISBN 9783527633180. https://books.google.com/books?id=jYI5jtMA2qgC&pg=PA259.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 "Kynurenines in the CNS: recent advances and new questions". Nature Reviews. Drug Discovery 12 (1): 64–82. January 2013. doi:10.1038/nrd3793. PMID 23237916.
- ↑ "School of Medicine Professor Wins University System of Maryland (USM) Board of Regents Award". University of Maryland. April 6, 2007. http://somvweb.som.umaryland.edu/absolutenm/templates/?a=143.
- ↑ "Press Release: VistaGen Therapeutics Acquires Artemis Neuroscience, Inc. - Enters Late-Stage Preclinical Development Program for Lead Epilepsy Drug Candidate -". PR Newswire. November 19, 2003. http://www.prnewswire.com/news-releases/vistagen-therapeutics-acquires-artemis-neuroscience-inc---enters-late-stage-preclinical-development-program-for-lead-epilepsy-drug-candidate---73081797.html.
- ↑ "VistaGen Therapeutics, Inc. 8-K Exhibit 10-26". SEC Edgar. May 16, 2011. https://www.sec.gov/Archives/edgar/data/1411685/000141588911000362/ex10-26.htm. See 8-K Index page at SEC Edgar.
- ↑ "VistaGen acquires Artemis Neuroscience". San Francisco Business Times. November 19, 2003. http://www.bizjournals.com/sanfrancisco/stories/2003/11/17/daily19.html.
- ↑ "Trial watch: phase II boost for glutamate-targeted antidepressants". Nature Reviews. Drug Discovery 12 (12): 897. December 2013. doi:10.1038/nrd4178. PMID 24287771.
- ↑ 10.0 10.1 "Emerging treatment mechanisms for depression: focus on glutamate and synaptic plasticity". Drug Discovery Today 21 (3): 454–464. March 2016. doi:10.1016/j.drudis.2016.01.016. PMID 26854424.
- ↑ "Rapid-acting antidepressant ketamine, its metabolites and other candidates: A historical overview and future perspective". Psychiatry and Clinical Neurosciences 73 (10): 613–627. October 2019. doi:10.1111/pcn.12902. PMID 31215725.
- ↑ "Characterization of the Effects of L-4-Chlorokynurenine on Nociception in Rodents" (in English). The Journal of Pain 18 (10): 1184–1196. October 2017. doi:10.1016/j.jpain.2017.03.014. PMID 28428091.
External links
- AV-101 - AdisInsight
- AV-101 - A Potential Breakthrough in Depression Treatment - VistaGen Therapeutics - YouTube
 |

