Chemistry:Tenocyclidine
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEMBL | |
| Chemical and physical data | |
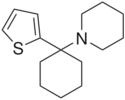
| Formula | C15H23NS |
| Molar mass | 249.42 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Tenocyclidine (TCP) is a dissociative anesthetic with psychostimulant effects. It was discovered by a team at Parke-Davis in the late 1950s.[1] It is similar in effects to phencyclidine (PCP) but is considerably more potent. TCP has slightly different binding properties to PCP, with more affinity for the NMDA receptors,[2] but less affinity for the sigma receptors.[3] Because of its high affinity for the PCP site of the NMDA receptor complex, the 3H radiolabelled form of TCP is widely used in research into NMDA receptors.
TCP acts primarily as an NMDA receptor antagonist which blocks the activity of the NMDA receptor, however its increased psychostimulant effects compared to PCP suggests it also has relatively greater activity as a dopamine reuptake inhibitor (DRI). Due to its similarity in effects to PCP, TCP was placed into the Schedule I list of illegal drugs in the 1970s, although it was only briefly used in the 1970s and 1980s and is now little known.[citation needed]
See also
- Arylcyclohexylamine
- Benocyclidine (BTCP)
References
- ↑ U.S. Patent 2,921,076 Heterocyclic compounds and methods for producing the same
- ↑ "The binding of [3H]thienyl cyclohexylpiperidine ([3H]TCP) to the NMDA-phencyclidine receptor complex". Neuropharmacology 28 (1): 1–7. January 1989. doi:10.1016/0028-3908(89)90059-2. PMID 2538766.
- ↑ "Non-competitive regulation of phencyclidine/sigma-receptors by the N-methyl-D-aspartate receptor antagonist D-(-)-2-amino-5-phosphonovaleric acid". Neuroscience Letters 78 (2): 193–8. July 1987. doi:10.1016/0304-3940(87)90632-x. PMID 2888059.
| Inhalational | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injection |
| ||||||||||||
| |||||||||||||
| |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
 | 0.00      (0 votes) (0 votes) |