Chemistry:PCPr
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
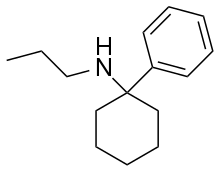
| Formula | C15H23N |
| Molar mass | 217.356 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
PCPr is an arylcyclohexylamine dissociative anesthetic drug with hallucinogenic and stimulant effects. It is around the same potency as phencyclidine, although slightly less potent than the ethyl homologue eticyclidine,[1] and has reportedly been sold as a designer drug in Germany and other European countries since the late 1990s.[2][3]
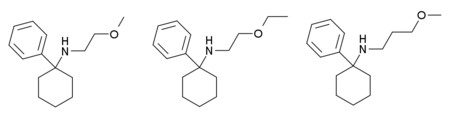
Several other related derivatives have also been encountered, with the n-propyl group of PCPr replaced by a 2-methoxyethyl, 2-ethoxyethyl or 3-methoxypropyl group to form PCMEA, PCEEA and PCMPA respectively.[4][5][6]
References
- ↑ "The Synthesis of Phencyclidine and Other 1-Arylcyclohexylamines". Journal of Medicinal Chemistry 8 (2): 230–5. March 1965. doi:10.1021/jm00326a019. PMID 14332667.
- ↑ "Metabolism and toxicological detection of a new designer drug, N-(1-phenylcyclohexyl)propanamine, in rat urine using gas chromatography-mass spectrometry". Journal of Chromatography A 1186 (1–2): 380–90. April 2008. doi:10.1016/j.chroma.2007.11.002. PMID 18035363.
- ↑ Christoph Sauer. Phencyclidine Derivatives – A new Class of Designer Drugs. Studies on the Metabolism and Toxicological Analysis. Universität des Saarlandes, 2008
- ↑ "New designer drugs N-(1-phenylcyclohexyl)-2-ethoxyethanamine (PCEEA) and N-(1-phenylcyclohexyl)-2-methoxyethanamine (PCMEA): Studies on their metabolism and toxicological detection in rat urine using gas chromatographic/mass spectrometric techniques". Journal of Mass Spectrometry 43 (3): 305–16. March 2008. doi:10.1002/jms.1312. PMID 17968862. Bibcode: 2008JMSp...43..305S.
- ↑ "Investigations on the cytochrome P450 (CYP) isoenzymes involved in the metabolism of the designer drugs N-(1-phenyl cyclohexyl)-2-ethoxyethanamine and N-(1-phenylcyclohexyl)-2-methoxyethanamine". Biochemical Pharmacology 77 (3): 444–50. February 2009. doi:10.1016/j.bcp.2008.10.024. PMID 19022226.
- ↑ "Metabolism and toxicological detection of the designer drug N-(1-phenylcyclohexyl)-3-methoxypropanamine (PCMPA) in rat urine using gas chromatography-mass spectrometry". Forensic Science International 181 (1–3): 47–51. October 2008. doi:10.1016/j.forsciint.2008.09.001. PMID 18922655.
 |
