Chemistry:Zelquistinel
 | |
| Clinical data | |
|---|---|
| Routes of administration | By mouth |
| Drug class | NMDA receptor modulator |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
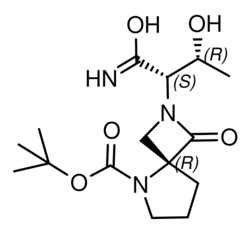
| Formula | C15H25N3O5 |
| Molar mass | 327.381 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Zelquistinel (GATE-251, formerly AGN-241751) is an orally active small-molecule NMDA receptor modulator which is under development for the treatment of major depressive disorder (MDD) by Gate Neurosciences, and previously by Allergan.[1][2][3]
Zelquistinel acts through a unique binding site on the NMDA receptor, independent of the glycine site, to modulate receptor activity and enhance NMDAR-mediated synaptic plasticity.[4][5] Its mechanism of action is similar to that of rapastinel. However, unlike rapastinel, zelquistinel is orally bioavailable, exhibits increased potency, and has improved drug properties.[2][3][5]
On July 23, 2018, the U.S. FDA granted Fast Track designation to the development of zelquistinel as an investigational new treatment for major depressive disorder.[6] As of August 2019, the drug had completed a phase IIa clinical trial.[1][3][7]
See also
References
- ↑ 1.0 1.1 "NMDA receptor modulators - AdisInsight". https://adisinsight.springer.com/drugs/800031388.
- ↑ 2.0 2.1 "Home - Gate Neurosciences" (in en-US). https://www.gateneuro.com/.
- ↑ 3.0 3.1 3.2 Aptinyx Inc.. "Allergan Exercises Option to Acquire Compound from Aptinyx Discovery Platform Under Ongoing Research Collaboration". https://www.prnewswire.com/news-releases/allergan-exercises-option-to-acquire-compound-from-aptinyx-discovery-platform-under-ongoing-research-collaboration-300652397.html.
- ↑ "Positive N-Methyl-D-Aspartate Receptor Modulation by Rapastinel Promotes Rapid and Sustained Antidepressant-Like Effects". The International Journal of Neuropsychopharmacology 22 (3): 247–259. March 2019. doi:10.1093/ijnp/pyy101. PMID 30544218.
- ↑ 5.0 5.1 "Positive modulation of NMDA receptors by AGN-241751 exerts rapid antidepressant-like effects via excitatory neurons". Neuropsychopharmacology 46 (4): 799–808. March 2021. doi:10.1038/s41386-020-00882-7. PMID 33059355.
- ↑ plc, Allergan. "Allergan Receives FDA Fast Track Designation for AGN-241751 for the Treatment of Major Depressive Disorder (MDD)" (in en). https://www.prnewswire.com/news-releases/allergan-receives-fda-fast-track-designation-for-agn-241751-for-the-treatment-of-major-depressive-disorder-mdd-300684622.html.
- ↑ Clinical trial number NCT03586427 for "A Double-Blind, Placebo-Controlled, Fixed-Dose Study of AGN-241751 in Adult Participants With Major Depressive Disorder" at ClinicalTrials.gov
External links

