Chemistry:Hematoporphyrin
From HandWiki
Short description: Chemical compound
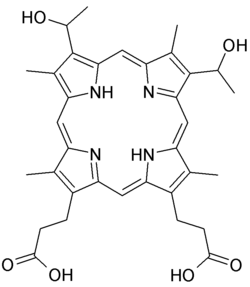 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C34H38N4O6 |
| Molar mass | 598.700 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 172.5 °C (342.5 °F) |
| |
| |
| (verify) | |
Hematoporphyrin (Photodyn, Sensibion) is a porphyrin prepared from hemin. It is a derivative of protoporphyrin IX, where the two vinyl groups have been hydrated (converted to alcohols). It is a deeply colored solid that is usually encountered as a solution. Its chemical structure was determined in 1900.[1]
It is used as a photosensitizer in photodynamic therapy. Acetylation of hematoporphyrin followed by hydrolysis of the product of that reaction affords a mixture called hematoporphyrin derivative (HPD), which is also used in photodynamic therapy.[2]
Hematoporphyrin has also been used as an antidepressant and antipsychotic since the 1920s.[3][4]
References
- ↑ "Hematoporphyrin IX". Pharmaceutical Chemistry Journal 11 (5): 613–20. May 1977. doi:10.1007/BF00780815. https://link.springer.com/article/10.1007%2FBF00780815.
- ↑ "Hematoporphyrin and HPD: photophysics, photochemistry and phototherapy". Photochemistry and Photobiology 39 (6): 851–9. June 1984. doi:10.1111/j.1751-1097.1984.tb08871.x. PMID 6235529.
- ↑ O'Neil, Maryadele J. (2001). The Merck index: an encyclopedia of chemicals, drugs, and biologicals. Rahway, NJ: Merck Research Laboratories. ISBN 0-911910-13-1. https://archive.org/details/merckindexency00onei.
- ↑ "Hematoporphyrin as a Therapeutic Agent in the Psychoses". American Journal of Psychiatry 90 (6): 1157–1173. May 1934. doi:10.1176/ajp.90.6.1157.
 |

