Chemistry:Iprindole
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prondol, Galatur, Tertran |
| Other names | Pramindole; WY-3263 |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic[3] |
| Elimination half-life | 52.5 hours[1] |
| Excretion | Urine, Feces[2] |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H28N2 |
| Molar mass | 284.447 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Iprindole, sold under the brand names Prondol, Galatur, and Tertran, is an atypical tricyclic antidepressant (TCA) that has been used in the United Kingdom and Ireland for the treatment of depression but appears to no longer be marketed.[4][5][6][7] It was developed by Wyeth and was marketed in 1967.[8] The drug has been described by some as the first "second-generation" antidepressant to be introduced.[9] However, it was very little-used compared to other TCAs, with the number of prescriptions dispensed only in the thousands.[10]
Medical uses
Iprindole was used in the treatment of major depressive disorder in dosages similar to those of other TCAs.[5][11]
Contraindications
Iprindole has been associated with jaundice and hepatotoxicity and should not be taken by alcoholics or people with pre-existing liver disease.[8][12][13][14] If such symptoms are encountered iprindole should be discontinued immediately.
Side effects
Anticholinergic side effects such as dry mouth and constipation are either greatly reduced in comparison to imipramine and most other TCAs or fully lacking with iprindole.[15] However, it still has significant antihistamine effects and therefore can produce sedation, though this is diminished relative to other TCAs similarly.[16] Iprindole also lacks significant alpha-blocking properties, and hence does not pose a risk of orthostatic hypotension.[16]
Overdose
In overdose, iprindole is much less toxic than most other TCAs and is considered relatively benign.[17] For instance, between 1974 and 1985, only two deaths associated with iprindole were recorded in the United Kingdom, whereas 278 were reported for imipramine, although imipramine is used far more often than iprindole.[10][17]
Interactions
Iprindole has been shown to be a potent inhibitor of the aromatic hydroxylation and/or N-dealkylation-mediated metabolism of many substances including, but not limited to octopamine, amphetamine, methamphetamine, fenfluramine, phenelzine, tranylcypromine, trimipramine, and fluoxetine, likely via inactivating cytochrome P450 enzymes.[3][18][19][20][21][22] It also inhibits its own metabolism.[21]
On account of these interactions, caution should be used when combining iprindole with other drugs.[3] As an example, when administered with amphetamine or methamphetamine, iprindole increases their brain concentrations and prolongs their terminal half-lives by 2- to 3-fold, strongly augmenting both their physiological effects and neurotoxicity in the process.[23][24][25]
Pharmacology
Pharmacodynamics
| Site | Ki (nM) | Species | Ref |
|---|---|---|---|
| NET | 1,262 | Human | [26] |
| DAT | 6,530 | Human | [26] |
| 5-HT1A | 2,800 | Human | [27] |
| 5-HT2A | 217–280 | Human/rat | [27][28] |
| 5-HT2C | 206 | Rat | [28] |
| α1 | 2,300 | Human | [29] |
| α2 | 8,600 | Human | [29] |
| β | >10,000 | Mammal | [30][31] |
| D2 | 6,300 | Rat | [31] |
| H1 | 100–130 | Human/rat | [29][32] |
| H2 | 200–8,300 | Guinea pig | [31][33][34] |
| σ1 | >10,000 | Rat | [35] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | |||
Iprindole is unique compared to most other TCAs in that it is a very weak and negligible inhibitor of the reuptake of serotonin and norepinephrine and appears to act instead as a selective albeit weak antagonist of 5-HT2 receptors; hence its classification by some as "second-generation".[36][37][38] Additionally, iprindole has very weak/negligible antiadrenergic and anticholinergic activity and weak although possibly significant antihistamine activity; as such, side effects of iprindole are much less prominent relative to other TCAs, and it is well tolerated.[15] However, iprindole may not be as effective as other TCAs, particularly in terms of anxiolysis.[36][16] Based on animal research, the antidepressant effects of iprindole may be mediated through downstream dopaminergic mechanisms.[39]
The binding affinities of iprindole for various biological targets are presented in the table to the right.[40] It is presumed to act as an inhibitor or antagonist/inverse agonist of all sites. Considering the range of its therapeutic concentrations (e.g., 63–271 nM at 90 mg/day),[1] only the actions of iprindole on the 5-HT2 and histamine receptors might be anticipated to be of possible clinical significance.[1] However, it is unknown whether these actions are in fact responsible for the antidepressant effects of iprindole. The plasma protein binding of iprindole and hence its free percentage and potentially bioactive concentrations do not seem to be known.
Pharmacokinetics
Only one study appears to have evaluated the pharmacokinetics of iprindole.[1][41] A single oral dose of 60 mg iprindole to healthy volunteers has been found to achieve mean peak plasma concentrations of 67.1 ng/mL (236 nmol/L) after 2 to 4 hours.[1] The mean terminal half-life of iprindole was 52.5 hours, which is notably much longer than that of other TCAs like amitriptyline and imipramine.[1] Following chronic treatment with 90 mg/day iprindole for 3 weeks, plasma concentrations of the drug ranged between 18 and 77 ng/mL (63–271 nmol/L).[1] Theoretical steady-state concentrations should be reached by 99% within 15 to 20 days of treatment.[1]
Chemistry
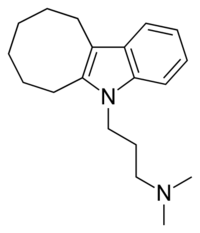
Iprindole is a tricyclic compound, specifically a cyclooctaindole (that is, an indole nucleus joined with a cyclooctyl ring), and possesses three rings fused together with a side chain attached in its chemical structure.[42] It is a tertiary amine TCA, although its ring system and pharmacological properties are very different from those of other TCAs.[15][43] Other tertiary amine TCAs that are similar to iprindole include butriptyline and trimipramine.[44][45] The chemical name of iprindole is 3-(6,7,8,9,10,11-hexahydro-5H-cycloocta[b]indol-5-yl)-N,N-dimethylpropan-1-amine and its free base form has a chemical formula of C19H28N2 with a molecular weight of 284.439 g/mol.[46] The drug has been used commercially as both the free base and the hydrochloride salt.[46] The CAS Registry Number of the free base is 5560-72-5 and of the hydrochloride is 20432-64-8.[46]
History
Iprindole was developed by Wyeth and was marketed in 1967.[8][47]
Society and culture
Generic names
Iprindole is the English and French generic name of the drug and its INN, USAN, BAN, and DCF, while iprindole hydrochloride is its BANM.[46][4][48] Its generic name in Spanish and German is iprindol while its generic name in Latin is iprindolum.[4] Iprindole was originally known unofficially as pramindole.[46][4]
Brand names
Iprindole has been marketed under the brand name Prondol by Wyeth in the United Kingdom and Ireland for the indication of major depressive disorder,[49] and has also been sold as Galatur and Tertran by Wyeth.[46]
Availability
Iprindole was previously available in the United Kingdom and Ireland[49] but seems to no longer be available for medical use in any country.[4]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 1.7 "Quantitation of iprindole in plasma by GLC". Biopharmaceutics & Drug Disposition 3 (1): 11–17. 1982. doi:10.1002/bdd.2510030103. PMID 7082775.
- ↑ "The disposition of [14C]iprindole in man, dog, miniature swine, rhesus monkey and rat". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 9 (4): 237–246. April 1979. doi:10.3109/00498257909038726. PMID 113942.
- ↑ 3.0 3.1 3.2 "Metabolism of some "second"- and "fourth"-generation antidepressants: iprindole, viloxazine, bupropion, mianserin, maprotiline, trazodone, nefazodone, and venlafaxine". Cellular and Molecular Neurobiology 19 (4): 427–442. August 1999. doi:10.1023/A:1006953923305. PMID 10379419.
- ↑ 4.0 4.1 4.2 4.3 4.4 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 569–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA569.
- ↑ 5.0 5.1 "Iprindole". Lexicon of psychiatry, neurology, and the neurosciences. Philadelphia, Pa: Lippincott-Williams & Wilkins. 2000. ISBN 0-7817-2468-6. https://books.google.com/books?id=ea_QVG2BFy8C&q=iprindole&pg=PA531.
- ↑ Dictionary of organic compounds. London: Chapman & Hall. 1996. ISBN 0-412-54090-8. https://books.google.com/books?id=x2Su3GKCvtsC&q=iprindole%20prondol&pg=PA3975.
- ↑ "Antidepressant and Biochemical Theories of Depression". Readings in Abnormal Psychology. New York: Wiley. 1989. p. 186. ISBN 0-471-63107-8. https://archive.org/details/isbn_9780471631071. "iprindole."
- ↑ 8.0 8.1 8.2 "Jaundice from iprindole (Prondol)". Drug and Therapeutics Bulletin 9 (3): 10–11. January 1971. doi:10.1136/dtb.9.3.10. PMID 5548547.
- ↑ "Second generation antidepressants: The pharmacological and clinical significance of selected examples". Drug Development Research 3 (3): 203–211. January 1983. doi:10.1002/ddr.430030302. https://onlinelibrary.wiley.com/doi/10.1002/ddr.430030302.
- ↑ 10.0 10.1 "Tricyclic Antidepressants". Meyler's Side Effects of Psychiatric Drugs. Elsevier. 2009. pp. 18–. ISBN 978-0-444-53266-4. https://books.google.com/books?id=AmYFTSO8jCkC&pg=PA18.
- ↑ "Treatment for Affective Disorders". Psychoses of uncertain aetiology. Cambridge, UK: Cambridge University Press. 1982. ISBN 0-521-28438-4. https://books.google.com/books?id=QFI4AAAAIAAJ&pg=PA167.
- ↑ Meyler's Side Effects of Psychiatric Drugs (Meylers Side Effects). Amsterdam: Elsevier Science. 2008. ISBN 978-0-444-53266-4. https://books.google.com/books?id=s0XYvuPVgaAC&q=iprindole&pg=PA87.
- ↑ "Jaundice due to iprindole". Gut 12 (9): 705–708. September 1971. doi:10.1136/gut.12.9.705. PMID 4106521.
- ↑ "Allergy to iprindole (Prondole) with hepatotoxicity". British Medical Journal 2 (5763): 712. June 1971. doi:10.1136/bmj.2.5763.712. PMID 5556082.
- ↑ 15.0 15.1 15.2 "Some recently introduced drugs". Progress in Medicinal Chemistry (Butterworth-Heinemann) 7 (1): 1–67 (25). 1970. doi:10.1016/s0079-6468(08)70351-5. ISBN 978-0-408-70013-9. PMID 4250600. https://books.google.com/books?id=hNcBVxpSfGUC&pg=PA25.
- ↑ 16.0 16.1 16.2 "Iprindole and imipramine in non-psychotic depressed out-patients". The British Journal of Psychiatry 123 (574): 329–339. September 1973. doi:10.1192/bjp.123.3.329. PMID 4583430.
- ↑ 17.0 17.1 "Fatal toxicity of antidepressant drugs in overdose". British Medical Journal 295 (6605): 1021–1024. October 1987. doi:10.1136/bmj.295.6605.1021. PMID 3690249.
- ↑ "The effects of imipramine and iprindole on the metabolism of octopamine in the rat". Neuropharmacology 24 (8): 705–708. August 1985. doi:10.1016/0028-3908(85)90002-4. PMID 3939325.
- ↑ "Interactions of iprindole with fenfluramine metabolism in rat brain and liver". Journal of Psychiatry & Neuroscience 16 (1): 5–11. March 1991. PMID 2049371.
- ↑ "Metabolism of methamphetamine, amphetamine and p-hydroxymethamphetamine by rat-liver microsomal preparations in vitro". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 14 (11): 867–875. November 1984. doi:10.3109/00498258409151485. PMID 6506759.
- ↑ 21.0 21.1 "Effect of iprindole on the metabolism of trimipramine in the rat". Journal of Psychiatry & Neuroscience 16 (5): 272–275. December 1991. PMID 1797102.
- ↑ "The effects of desipramine and iprindole on levels of enantiomers of fluoxetine in rat brain and urine". Chirality 6 (2): 86–90. 1994. doi:10.1002/chir.530060208. PMID 8204417.
- ↑ "Biological disposition of rigid analogs of amphetamine". Journal of Pharmaceutical Sciences 66 (2): 271–272. February 1977. doi:10.1002/jps.2600660235. PMID 839428.
- ↑ "Long-lasting depletion of striatal dopamine by a single injection of amphetamine in iprindole-treated rats". Science 209 (4453): 305–307. July 1980. doi:10.1126/science.7384808. PMID 7384808. Bibcode: 1980Sci...209..305F.
- ↑ "Effects of a single dose of methamphetamine and iprindole on the serotonergic and dopaminergic system of the rat brain". The Journal of Pharmacology and Experimental Therapeutics 225 (1): 126–131. April 1983. PMID 6187915. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=6187915.
- ↑ 26.0 26.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9537821 - ↑ 27.0 27.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid3816971 - ↑ 28.0 28.1 "Interactions of selective serotonin reuptake inhibitors with the serotonin 5-HT2c receptor". Psychopharmacology 126 (3): 234–240. August 1996. doi:10.1007/bf02246453. PMID 8876023.
- ↑ 29.0 29.1 29.2 "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". The Journal of Pharmacology and Experimental Therapeutics 230 (1): 94–102. July 1984. PMID 6086881.
- ↑ "Beta adrenergic receptor binding in membrane preparations from mammalian brain". Molecular Pharmacology 12 (4): 568–580. July 1976. PMID 8699.
- ↑ 31.0 31.1 31.2 "In Vitro and Ex Vivo Neurochemical Screening Procedures for Antidepressants, Neuroleptics, and Benzodiazepines". Analysis of Psychiatric Drugs. 10. 1988. pp. 327–378. doi:10.1385/0-89603-121-7:327. ISBN 0-89603-121-7.
- ↑ "Histamine H1 receptors identified in mammalian brain membranes with [3Hmepyramine"]. Proceedings of the National Academy of Sciences of the United States of America 75 (12): 6290–6294. December 1978. doi:10.1073/pnas.75.12.6290. PMID 282646. Bibcode: 1978PNAS...75.6290T.
- ↑ "Differences in the interaction of histamine H2 receptor antagonists and tricyclic antidepressants with adenylate cyclase from guinea pig gastric mucosa". Biochemical Pharmacology 33 (22): 3621–3625. November 1984. doi:10.1016/0006-2952(84)90147-3. PMID 6150708.
- ↑ "Antidepressants are weak competitive antagonists of histamine H2 receptors in dissociated brain tissue". European Journal of Pharmacology 94 (3–4): 313–318. October 1983. doi:10.1016/0014-2999(83)90420-x. PMID 6140176.
- ↑ "Psychotomimetic opiate receptors labeled and visualized with (+)-[3H3-(3-hydroxyphenyl)-N-(1-propyl)piperidine"]. Proceedings of the National Academy of Sciences of the United States of America 81 (15): 4983–4987. August 1984. doi:10.1073/pnas.81.15.4983. PMID 6087359. Bibcode: 1984PNAS...81.4983L.
- ↑ 36.0 36.1 "Novel antidepressants and the biogenic amine hypothesis of depression. The case for iprindole and mianserin". Archives of General Psychiatry 36 (10): 1097–1107. September 1979. doi:10.1001/archpsyc.1979.01780100067006. PMID 475543.
- ↑ "Comparative pharmacological studies on butriptyline and some related standard tricyclic antidepressants". Canadian Journal of Physiology and Pharmacology 53 (1): 104–112. February 1975. doi:10.1139/y75-014. PMID 166748.
- ↑ "Structure-activity relations for the inhibition of 5-hydroxytryptamine uptake by tricyclic antidepressants into synaptosomes from serotoninergic neurones in rat brain homogenates". British Journal of Pharmacology 51 (3): 399–403. July 1974. doi:10.1111/j.1476-5381.1974.tb10675.x. PMID 4451753.
- ↑ "Chronic treatment with iprindole reduces immobility of rats in the behavioural 'despair' test by activating dopaminergic mechanisms in the brain". The Journal of Pharmacy and Pharmacology 38 (4): 313–315. April 1986. doi:10.1111/j.2042-7158.1986.tb04576.x. PMID 2872301.
- ↑ Cite error: Invalid
<ref>tag; no text was provided for refs namedPDSP - ↑ "Pharmacokinetic optimisation of therapy with newer antidepressants". Clinical Pharmacokinetics 27 (4): 307–330. October 1994. doi:10.2165/00003088-199427040-00005. PMID 7834966.
- ↑ "Tricyclic Antidepressants". Drugs in Psychiatric Practice. Elsevier. 22 October 2013. pp. 195–. ISBN 978-1-4831-9193-5. https://books.google.com/books?id=6gglBQAAQBAJ&pg=PA195.
- ↑ "Iprindole: an antidepressant which does not block REM sleep". Nature 223 (5207): 750–752. August 1969. doi:10.1038/223750a0. PMID 4308422. Bibcode: 1969Natur.223..750B.
- ↑ "Drugs that Affect the Central Nervous System". Pharmacology Secrets. Elsevier Health Sciences. 2002. pp. 39–. ISBN 1-56053-470-2. https://books.google.com/books?id=_QQsj3PAUrEC&pg=PA39.
- ↑ "Drugs and other physical treatments". Shorter Oxford Textbook of Psychiatry. OUP Oxford. 9 August 2012. pp. 532–. ISBN 978-0-19-162675-3. https://books.google.com/books?id=Y1DtSGq-LnoC&pg=PA532.
- ↑ 46.0 46.1 46.2 46.3 46.4 46.5 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 702–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA702.
- ↑ "Cyclic Antidepressant Drugs". Medical Toxicology. Lippincott Williams & Wilkins. 2004. pp. 836–. ISBN 978-0-7817-2845-4. https://books.google.com/books?id=BfdighlyGiwC&pg=PA836.
- ↑ Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 156–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA156.
- ↑ 49.0 49.1 Martindale: The Complete Drug Reference (36th ed.). London: Pharmaceutical Press. 2009. ISBN 978-0-85369-840-1.
Further reading
- "Iprindole: a cornerstone in the neurobiological investigation of antidepressant treatments". Modern Problems of Pharmacopsychiatry. Modern Trends in Pharmacopsychiatry 18: 102–116. 1982. doi:10.1159/000406238. ISBN 978-3-8055-3428-4. PMID 6285182.
- "Second generation antidepressants: The pharmacological and clinical significance of selected examples". Drug Development Research 3 (3): 203–211. January 1983. doi:10.1002/ddr.430030302. https://onlinelibrary.wiley.com/doi/10.1002/ddr.430030302.
 |
