Chemistry:Dosulepin
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Prothiaden, others |
| Other names | IZ-914, KS-1596[1][2][3], dothiepin (USAN US) |
| AHFS/Drugs.com | International Drug Names |
| Pregnancy category |
|
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 30%[4] |
| Protein binding | 84%[5] |
| Metabolism | Hepatic (N-demethylation, S-oxidation, glucuronidation)[5] |
| Metabolites | Northiaden, dothiepin sulfoxide, northiaden sulfoxide, glucuronide conjugates[4] |
| Elimination half-life | Dothiepin: 14.4–23.9 hours[4] Dothiepin sulfoxide: 22.7–25.5 hours[4] Northiaden: 34.7–45.7 hours[4] Northiaden sulfoxide: 24.2–33.5 hours[4] |
| Excretion | Urine: 56%[4] Feces: 15%[4] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII |
|
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C19H21NS |
| Molar mass | 295.44 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Dosulepin, also known as dothiepin and sold under the brand name Prothiaden among others, is a tricyclic antidepressant (TCA) which is used in the treatment of depression.[4][6][7] Dosulepin was once the most frequently prescribed antidepressant in the United Kingdom , but it is no longer widely used due to its relatively high toxicity in overdose without therapeutic advantages over other TCAs.[6][8][9] It acts as a serotonin–norepinephrine reuptake inhibitor (SNRI) and also has other activities including antihistamine, antiadrenergic, antiserotonergic, anticholinergic, and sodium channel-blocking effects.[4][10][11]
Medical uses
Dosulepin is used for the treatment of major depressive disorder.[4][5][12][13] There is clear evidence of the efficacy of dosulepin in psychogenic facial pain, though the drug may be needed for up to a year.[14]
Contraindications
Contraindications include:[5]
- Epilepsy as it can lower the seizure threshold
- TCAs should not be used concomitantly or within 14 days of treatment with monoamine oxidase inhibitors due to the risk for serotonin syndrome
- Acute recovery phase following myocardial infarction as TCAs may produce conduction defects and arrhythmias
- Liver failure
- Hypersensitivity to dosulepin
Side effects
Common adverse effects:[5]
- Drowsiness
- Extrapyramidal symptoms
- Tremor
- Disorientation
- Dizziness
- Paresthesias
- Alterations to ECG patterns
- Dry mouth
- Sweating
- Urinary retention
- Hypotension
- Postural hypotension
- Tachycardia
- Palpitations
- Arrhythmias
- Conduction defects
- Increased or decreased libido
- Nausea
- Vomiting
- Constipation
- Blurred vision
Less common adverse effects:[5]
- Disturbed concentration
- Delusions
- Hallucinations
- Anxiety
- Fatigue
- Headaches
- Restlessness
- Excitement
- Insomnia
- Hypomania
- Nightmares
- Peripheral neuropathy
- Ataxia
- Incoordination
- Seizures
- Paralytic ileus
- Hypertension
- Heart block
- Myocardial infarction
- Stroke
- Gynecomastia (swelling of breast tissue in males)
- Testicular swelling
- Impotence
- Epigastric distress
- Abdominal cramps
- Parotid swellings
- Diarrhea
- Stomatitis (swelling of the mouth)
- Black tongue
- Peculiar taste sensations
- Cholestatic jaundice
- Altered liver function
- Hepatitis (swelling of the liver)
- Skin rash
- Urticaria (hives)
- Photosensitisation
- Skin blisters
- Angioneurotic edema
- Weight loss
- Urinary frequency
- Mydriasis
- Weight gain
- Hyponatremia (low blood sodium)
- Movement disorders
- Dyspepsia (indigestion)
- Increased intraocular pressure
- Changes in blood sugar levels
- Thrombocytopenia (an abnormally low number of platelets in the blood. This makes one more susceptible to bleeds)
- Eosinophilia (an abnormally high number of eosinophils in the blood)
- Agranulocytosis (a dangerously low number of white blood cells in the blood leaving one open to potentially life-threatening infections)
- Galactorrhea (lactation that is unassociated with breastfeeding and lactation)
Overdose
The symptoms and the treatment of an overdose are largely the same as for the other TCAs.[12] Dosulepin may be particularly toxic in overdose compared to other TCAs.[12] The onset of toxic effects is around 4–6 hours after dosulepin is ingested.[5] In order to minimise the risk of overdose it is advised that patients only receive a limited number of tablets at a time so as to limit their risk of overdosing.[5] It is also advised that patients are not prescribed any medications that are known to increase the risk of toxicity in those receiving dosulepin due to the potential for mixed overdoses.[5] The medication should also be kept out of reach of children.[5]
Interactions
Dosulepin can potentiate the effects of alcohol and at least one death has been attributed to this combination.[5] TCAs potentiate the sedative effects of barbiturates, tranquilizers and CNS depressants.[5] Guanethidine and other adrenergic neuron blocking drugs can have their antihypertensive effects blocked by dosulepin.[5] Sympathomimetics may potentiate the sympathomimetic effects of dosulepin.[5] Due to the anticholinergic and antihistamine effects of dosulepin anticholinergic and antihistamine medications may have their effects potentiated by dosulepin and hence these combinations are advised against.[5] Dosulepin may have its postural hypotensive effects potentiated by diuretics.[5] Anticonvulsants may have their efficacy reduced by dosulepin due to its ability to reduce the seizure threshold.[5]
Pharmacology
Pharmacodynamics
| Site | DSP | NTD | Species | Ref |
|---|---|---|---|---|
| NET | 46–70 | 25 | Human/rat | [15][11] |
| DAT | 5,310 | 2,539 | Human/rat | [15][11] |
| 5-HT1A | 4,004 | 2,623 | Rat | [16] |
| 5-HT2A | 152 | 141 | Rat | [11] |
| α1 | 419 | 950 | Rat | [11] |
| α2 | 2,400 | ND | Human | [17] |
| H1 | 3.6–4 | 25 | Human/rat | [11][17] |
| M1 | 18 | ND | Human | [18] |
| M2 | 109 | ND | Human | [18] |
| M3 | 38 | ND | Human | [18] |
| M4 | 61 | ND | Human | [18] |
| M5 | 92 | ND | Human | [18] |
| Values are Ki (nM). The smaller the value, the more strongly the drug binds to the site. | ||||
Dosulepin is a reuptake inhibitor of the serotonin transporter (SERT) and the norepinephrine transporter (NET), thereby acting as an SNRI.[11][10] It is also an antagonist of the histamine H1 receptor, α1-adrenergic receptor, serotonin 5-HT2 receptors, and muscarinic acetylcholine receptors (mACh), as well as a blocker of voltage-gated sodium channels (VGSCs).[11][4] The antidepressant effects of dosulepin are thought to be due to inhibition of the reuptake of norepinephrine and possibly also of serotonin.[4]
Dosulepin has three metabolites, northiaden (desmethyldosulepin), dosulepin sulfoxide, and northiaden sulfoxide, which have longer terminal half-lives than that of dosulepin itself.[11] However, whereas northiaden has potent activity similarly to dosulepin, the two sulfoxide metabolites have dramatically reduced activity.[11] They have been described as essentially inactive, and are considered unlikely to contribute to either the therapeutic effects or side effects of dosulepin.[11] Relative to dosulepin, northiaden has reduced activity as a serotonin reuptake inhibitor, antihistamine, and anticholinergic and greater potency as a norepinephrine reuptake inhibitor,[11] similarly to other secondary amine TCAs.[19][20] Unlike the sulfoxide metabolites, northiaden is thought to play an important role in the effects of dosulepin.[11]
Although Heal & Cheetham (1992) reported relatively high Ki values of 12 and 15 nM for dosulepin and northiaden at the rat α2-adrenergic receptor and suggested that antagonism of the receptor could be involved in the antidepressant effects of dosulepin,[11] Richelson & Nelson (1984) found a low KD of only 2,400 nM for dosulepin at this receptor using human brain tissue.[17] This suggests that it in fact has low potency for this action, similarly to other TCAs.[17]
Pharmacokinetics
Dosulepin is readily absorbed from the small intestine and is extensively metabolized on first-pass through the liver into its chief active metabolite, northiaden.[5] Peak plasma concentrations of between 30.4 and 279 ng/mL (103–944 nmol/L) occur within 2–3 hours of oral administration.[5] It is distributed in breast milk and crosses the placenta and blood–brain barrier.[5] It is highly bound to plasma proteins (84%), and has a whole-body elimination half-life of 51 hours.[5]
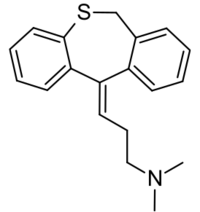
Chemistry
Dosulepin is a tricyclic compound, specifically a dibenzothiepine, and possesses three rings fused together with a side chain attached in its chemical structure.[21] It is the only TCA with a dibenzothiepine ring system to have been marketed.[21][22] The drug is a tertiary amine TCA, with its side chain-demethylated metabolite northiaden (desmethyldosulepin) being a secondary amine.[23][24] Other tertiary amine TCAs include amitriptyline, imipramine, clomipramine, doxepin, and trimipramine.[25][26] Dosulepin exhibits (E) and (Z) stereoisomerism like doxepin but in contrast the pure E or trans isomer is used medicinally.[1][10][27] The drug is used commercially as the hydrochloride salt; the free base is not used.
History
Dosulepin was developed by SPOFA.[28] It was patented in 1962 and first appeared in the literature in 1962.[28] The drug was first introduced for medical use in 1969, in the United Kingdom .[28][29]
Society and culture
Generic names
Dosulepin is the English and German generic name of the drug and its INN and BAN, while dosulepin hydrochloride is its BANM and JAN.[1][2][30][3] Dothiepin is the former BAN of the drug while dothiepin hydrochloride is the former BANM and remains the current USAN.[1][2][30][3] Its generic name in Spanish and Italian and its DCIT are dosulepina, in French and its DCF are dosulépine, and in Latin is dosulepinum.[2][3]
Brand names
Dosulepin is marketed throughout the world mainly under the brand name Prothiaden.[2][3] It is or has been marketed under a variety of other brand names as well, including Altapin, Depresym, Dopress, Dothapax, Dothep, Idom, Prepadine, Protiaden, Protiadene, Thaden, and Xerenal.[1][30][2][3]
Availability
Dosulepin is marketed throughout Europe (as Prothiaden, Protiaden, and Protiadene), Australia (as Dothep and Prothiaden), New Zealand (as Dopress) and South Africa (as Thaden).[2][3][7][12][13] It is also available in Japan , Hong Kong, Taiwan, India , Singapore, and Malaysia.[2][3][7] The drug is not available in the United States or Canada .[2][3][7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 468–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA468.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 2.7 2.8 Index Nominum 2000: International Drug Directory. Taylor & Francis. 2000. pp. 369–. ISBN 978-3-88763-075-1. https://books.google.com/books?id=5GpcTQD_L2oC&pg=PA369.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 3.7 3.8 "Dosulepin". https://www.drugs.com/international/dosulepin.html.
- ↑ 4.00 4.01 4.02 4.03 4.04 4.05 4.06 4.07 4.08 4.09 4.10 4.11 4.12 "Dothiepin. A review of its pharmacodynamic and pharmacokinetic properties, and therapeutic efficacy in depressive illness". Drugs 38 (1): 123–47. 1989. doi:10.2165/00003495-198938010-00005. PMID 2670509.
- ↑ 5.00 5.01 5.02 5.03 5.04 5.05 5.06 5.07 5.08 5.09 5.10 5.11 5.12 5.13 5.14 5.15 5.16 5.17 5.18 5.19 5.20 "Dothep Dothiepin hydrochloride" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 1 November 2013. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-04551-3.
- ↑ 6.0 6.1 "The tolerability of dothiepin: a review of clinical studies between 1963 and 1990 in over 13,000 depressed patients". Prog. Neuropsychopharmacol. Biol. Psychiatry 18 (7): 1143–62. 1994. doi:10.1016/0278-5846(94)90117-1. PMID 7846285.
- ↑ 7.0 7.1 7.2 7.3 Dosulepin Hydrochloride. London, UK: Pharmaceutical Press. 5 December 2011. http://www.medicinescomplete.com/mc/martindale/current/2512-p.htm. Retrieved 15 August 2017.
- ↑ "Tricyclic antidepressant poisoning : cardiovascular toxicity". Toxicol Rev 24 (3): 205–14. 2005. doi:10.2165/00139709-200524030-00013. PMID 16390222.
- ↑ "Tricyclic antidepressant pharmacology and therapeutic drug interactions updated". Br. J. Pharmacol. 151 (6): 737–48. 2007. doi:10.1038/sj.bjp.0707253. PMID 17471183.
- ↑ 10.0 10.1 10.2 Foye's Principles of Medicinal Chemistry. Lippincott Williams & Wilkins. 24 January 2012. pp. 607–. ISBN 978-1-60913-345-0. https://books.google.com/books?id=Sd6ot9ul-bUC&pg=PA607.
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 Cite error: Invalid
<ref>tag; no text was provided for refs namedHealCheetham1992 - ↑ 12.0 12.1 12.2 12.3 Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 978-0-9805790-9-3.
- ↑ 13.0 13.1 Joint Formulary Committee (2013). British National Formulary (BNF) (65 ed.). London, UK: Pharmaceutical Press. ISBN 978-0-85711-084-8. https://archive.org/details/bnf65britishnati0000unse.
- ↑ "Psychogenic facial pain: presentation and treatment". British Medical Journal 288 (6415): 436–438. February 1984. doi:10.1136/bmj.288.6415.436. PMID 6419955.
- ↑ 15.0 15.1 Cite error: Invalid
<ref>tag; no text was provided for refs namedpmid9537821 - ↑ "Comparison of the effects of antidepressants and their metabolites on reuptake of biogenic amines and on receptor binding". Cell. Mol. Neurobiol. 19 (4): 467–89. 1999. doi:10.1023/A:1006986824213. PMID 10379421.
- ↑ 17.0 17.1 17.2 17.3 "Antagonism by antidepressants of neurotransmitter receptors of normal human brain in vitro". J. Pharmacol. Exp. Ther. 230 (1): 94–102. 1984. PMID 6086881.
- ↑ 18.0 18.1 18.2 18.3 18.4 "Antagonism of the five cloned human muscarinic cholinergic receptors expressed in CHO-K1 cells by antidepressants and antihistaminics". Biochem. Pharmacol. 45 (11): 2352–4. 1993. doi:10.1016/0006-2952(93)90211-e. PMID 8100134.
- ↑ Essentials of Psychiatry. American Psychiatric Pub. 2011. pp. 468–. ISBN 978-1-58562-933-6. https://books.google.com/books?id=Hf50vMMMv_wC&pg=PA468.
- ↑ Tietz Textbook of Clinical Chemistry and Molecular Diagnostics - E-Book. Elsevier Health Sciences. 14 October 2012. pp. 1129–. ISBN 978-1-4557-5942-2. https://books.google.com/books?id=BBLRUI4aHhkC&pg=PA1129.
- ↑ 21.0 21.1 Meyler's Side Effects of Psychiatric Drugs. Elsevier. 2009. pp. 7–. ISBN 978-0-444-53266-4. https://books.google.com/books?id=AmYFTSO8jCkC&pg=PA7.
- ↑ Polypharmacy in Psychiatry Practice, Volume I: Multiple Medication Use Strategies. Springer Science & Business Media. 15 February 2013. pp. 270–271. ISBN 978-94-007-5805-6. https://books.google.com/books?id=jy-LMZU7338C&pg=PA270.
- ↑ Pharmacodynamics and Drug Development: Perspectives in Clinical Pharmacology. John Wiley & Sons. 20 September 1994. pp. 160–. ISBN 978-0-471-95052-3. https://books.google.com/books?id=ncRXa8Dq88QC&pg=PA160.
- ↑ Metabolism of Drugs and Other Xenobiotics. John Wiley & Sons. 23 February 2012. pp. 302–. ISBN 978-3-527-64632-6. https://books.google.com/books?id=f-XHh17NfwgC&pg=PA302.
- ↑ Pharmacology Secrets. Elsevier Health Sciences. 2002. pp. 39–. ISBN 1-56053-470-2. https://books.google.com/books?id=_QQsj3PAUrEC&pg=PA39.
- ↑ Shorter Oxford Textbook of Psychiatry. OUP Oxford. 9 August 2012. pp. 532–. ISBN 978-0-19-162675-3. https://books.google.com/books?id=Y1DtSGq-LnoC&pg=PA532.
- ↑ Psychotropic Agents: Part I: Antipsychotics and Antidepressants. Springer Science & Business Media. 6 December 2012. pp. 354–. ISBN 978-3-642-67538-6. https://books.google.com/books?id=oK7tCAAAQBAJ&pg=PA354.
- ↑ 28.0 28.1 28.2 "Recent advances in the understanding of the interaction of antidepressant drugs with serotonin and norepinephrine transporters". Chem. Commun. (25): 3677–92. 2009. doi:10.1039/b903035m. PMID 19557250.
- ↑ Medical Toxicology. Lippincott Williams & Wilkins. 2004. pp. 836–. ISBN 978-0-7817-2845-4. https://books.google.com/books?id=BfdighlyGiwC&pg=PA836.
- ↑ 30.0 30.1 30.2 Concise Dictionary of Pharmacological Agents: Properties and Synonyms. Springer Science & Business Media. 6 December 2012. pp. 105–. ISBN 978-94-011-4439-1. https://books.google.com/books?id=tsjrCAAAQBAJ&pg=PA105.
 |
