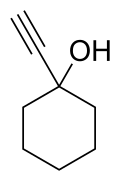
Chemistry:1-Ethynylcyclohexanol
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C8H12O |
| Molar mass | 124.183 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 30–33 °C (86–91 °F) |
| |
| |
1-Ethynylcyclohexanol (ECX) is an alkynyl alcohol derivative which is both a synthetic precursor to, and an active metabolite of the tranquilizer ethinamate, and has similar sedative, anticonvulsant and muscle relaxant effects. It has been sold as a designer drug, first being identified in the UK in March 2012.[1][2][3][4]
Preparation
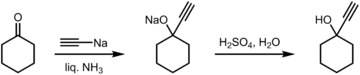
1-Ethynylcyclohexanol can be prepared from cyclohexanone by the reacting it with sodium acetylide in liquid ammonia, followed by an acidic work-up.[5]
See also
- 1,6-Dioxecane-2,7-dione
- 2-Methyl-2-butanol
- 2-Methyl-2-pentanol
- 3-Methyl-3-pentanol
- Clocental
- Ethchlorvynol
- Methylpentynol
- Prenderol
References
- ↑ "Action of sodium acetylide on cyclic ketones. I. Synthesis of 1-ethynylcyclohexanol.". Zhurnal Prikladnoi Khimii (Sankt-Peterburg, Russian Federation) 9: 1299–1302. 1936. ISSN 0044-4618.
- ↑ "An efficient and quick laboratory scale method for the ethynylation of some aliphatic and cycloaliphatic carbonyl compounds.". Synthetic Communications 18 (2): 131–4. February 1988. doi:10.1080/00397918808077336.
- ↑ "Acetylene derivatives. CLX. Condensation of aldehydes and ketones with acetylene under pressure. New method of synthesis of acetylenic alcohols.". Zhurnal Obshchei Khimii 23: 1900–1904. 1953. ISSN 0044-460X.
- ↑ "Europol 2012 Annual Report on the implementation of Council Decision 2005/387/JHA (New drugs in Europe, 2012)". Lisbon: EMCDDA. May 2013. https://www.emcdda.europa.eu/system/files/publications/734/EMCDDA-Europol_2012_Annual_Report_final_439477.pdf.
- ↑ "1-ETHYNYLCYCLOHEXANOL". Organic Syntheses 29: 47. 1949. doi:10.15227/orgsyn.029.0047. http://orgsyn.org/demo.aspx?prep=CV3P0416.
 |