Biology:GABAB receptor
| gamma-aminobutyric acid (GABA) B receptor, 1 | |
|---|---|
| Identifiers | |
| Symbol | GABBR1 |
| NCBI gene | 2550 |
| HGNC | 4070 |
| OMIM | 603540 |
| RefSeq | NM_021905 |
| UniProt | Q9UBS5 |
| Other data | |
| Locus | Chr. 6 p21.3 |
| gamma-aminobutyric acid (GABA) B receptor, 2 | |
|---|---|
| Identifiers | |
| Symbol | GABBR2 |
| Alt. symbols | GPR51 |
| NCBI gene | 9568 |
| HGNC | 4507 |
| OMIM | 607340 |
| RefSeq | NM_005458 |
| UniProt | O75899 |
| Other data | |
| Locus | Chr. 9 q22.1-22.3 |
GABAB receptors (GABABR) are G-protein coupled receptors for gamma-aminobutyric acid (GABA), therefore making them metabotropic receptors, that are linked via G-proteins to potassium channels.[1] The changing potassium concentrations hyperpolarize the cell at the end of an action potential. The reversal potential of the GABAB-mediated IPSP (inhibitory postsynaptic potential) is –100 mV, which is much more hyperpolarized than the GABAA IPSP. GABAB receptors are found in the central nervous system and the autonomic division of the peripheral nervous system.[2]
The receptors were first named in 1981 when their distribution in the CNS was determined, which was determined by Norman Bowery and his team using radioactively labelled baclofen.[3]
Functions
GABABRs stimulate the opening of K+ channels, specifically GIRKs, which brings the neuron closer to the equilibrium potential of K+. This reduces the frequency of action potentials which reduces neurotransmitter release.[citation needed] Thus GABAB receptors are inhibitory receptors.
GABAB receptors also reduces the activity of adenylyl cyclase and Ca2+ channels by using G-proteins with Gi/G0 α subunits.[4]
GABAB receptors are involved in behavioral actions of ethanol,[5][6] gamma-hydroxybutyric acid (GHB),[7] and possibly in pain.[8] Recent research suggests that these receptors may play an important developmental role.[9]

Structure
GABAB Receptors are similar in structure to and in the same receptor family with metabotropic glutamate receptors.[10] There are two subunits of the receptor, GABAB1 and GABAB2,[11] and these appear to assemble as obligate heterodimers in neuronal membranes by linking up by their intracellular C termini.[10] In the mammalian brain, two predominant, differentially expressed isoforms of the GABAB1 are transcribed from the Gabbr1 gene, GABAB(1a) and GABAB(1b), which are conserved in different species including humans.[12] This might potentially offer more complexity in terms of the function due to different composition of the receptor.[12] Cryo-electron microscopy structures of the full length GABAB receptor in different conformational states from inactive apo to fully active have been obtained. Unlike Class A and B GPCRs, phospholipids bind within the transmembrane bundles and allosteric modulators bind at the interface of GABAB1 and GABAB2 subunits.[13][14][15][16][17][18][19]
Ligands

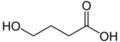

Agonists
- GABA
- Baclofen is a GABA analogue which acts as a selective agonist of GABAB receptors, and is used as a muscle relaxant. However, it can aggravate absence seizures, and so is not used in epilepsy.
- gamma-Hydroxybutyrate (GHB)
- Phenibut
- 4-Fluorophenibut
- Isovaline
- 3-Aminopropylphosphinic acid
- Lesogaberan
- SKF-97541: 3-Aminopropyl(methyl)phosphinic acid, 10x more potent than baclofen as GABAB agonist, but also GABAA-rho antagonist
- Taurine
- CGP-44532

Positive Allosteric Modulators


Antagonists
- Homotaurine[24]
- Ginsenosides[25]
- 2-OH-saclofen
- Saclofen
- Phaclofen
- SCH-50911
- 2-Phenethylamine
- CGP-35348
- CGP-52432: 3-([(3,4-Dichlorophenyl)methyl]amino]propyl) diethoxymethyl)phosphinic acid, CAS# 139667-74-6
- CGP-55845: (2S)-3-([(1S)-1-(3,4-Dichlorophenyl)ethyl]amino-2-hydroxypropyl)(phenylmethyl)phosphinic acid, CAS# 149184-22-5
- SGS-742[26][27]
See also
- GABA receptor
- GABAA receptor
- GABAA-ρ receptor
References
- ↑ "Role of GABAB receptors in GABA and baclofen-induced inhibition of adult rat cerebellar interpositus nucleus neurons in vitro". Brain Research Bulletin 67 (4): 310–8. October 2005. doi:10.1016/j.brainresbull.2005.07.004. PMID 16182939.
- ↑ "A Gut Feeling about GABA: Focus on GABA(B) Receptors". Frontiers in Pharmacology 1: 124. 2010. doi:10.3389/fphar.2010.00124. PMID 21833169.
- ↑ "3H-baclofen and 3H-GABA bind to bicuculline-insensitive GABA B sites in rat brain". Nature 290 (5802): 149–52. March 1981. doi:10.1038/290149a0. PMID 6259535. Bibcode: 1981Natur.290..149H.
- ↑ Rang and Dale's Pharmacology (8th ed.). Elsevier, Churchill Livingstone. 2016. pp. 462. ISBN 978-0-7020-5362-7. OCLC 903234097.
- ↑ "Gamma-aminobutyric acid B receptor 1 mediates behavior-impairing actions of alcohol in Drosophila: adult RNA interference and pharmacological evidence". Proceedings of the National Academy of Sciences of the United States of America 100 (9): 5485–90. April 2003. doi:10.1073/pnas.0830111100. PMID 12692303. Bibcode: 2003PNAS..100.5485D.
- ↑ "Ethanol potentiation of GABAergic synaptic transmission may be self-limiting: role of presynaptic GABA(B) receptors". The Journal of Neuroscience 24 (47): 10679–86. November 2004. doi:10.1523/JNEUROSCI.1768-04.2004. PMID 15564584.
- ↑ "Drosophila GABA(B) receptors are involved in behavioral effects of gamma-hydroxybutyric acid (GHB)". European Journal of Pharmacology 519 (3): 246–52. September 2005. doi:10.1016/j.ejphar.2005.07.016. PMID 16129424.
- ↑ "Drosophila model for in vivo pharmacological analgesia research". European Journal of Pharmacology 491 (2–3): 207–8. May 2004. doi:10.1016/j.ejphar.2004.03.030. PMID 15140638.
- ↑ "Developmental role of GABAB(1) receptors in Drosophila". Brain Research. Developmental Brain Research 158 (1–2): 111–4. August 2005. doi:10.1016/j.devbrainres.2005.06.005. PMID 16054235.
- ↑ 10.0 10.1 MRC (Medical Research Council). 2003. Glutamate receptors: Structures and functions. University of Brisotol Centre for Synaptic Plasticity.
- ↑ "7. Neurotransmitter Receptors and Their Effects". Neuroscience (Second ed.). Sinauer Associates, Inc. 2001. https://www.ncbi.nlm.nih.gov/books/NBK11099/.
- ↑ 12.0 12.1 "Expression cloning of GABA(B) receptors uncovers similarity to metabotropic glutamate receptors". Nature 386 (6622): 239–46. March 1997. doi:10.1038/386239a0. PMID 9069281. Bibcode: 1997Natur.386..239K. https://www.nature.com/articles/386239a0.
- ↑ "Molecular mechanisms of metabotropic GABAB receptor function". Science Advances 7 (22): eabg3362. May 2021. doi:10.1126/sciadv.abg3362. PMID 34049877. Bibcode: 2021SciA....7.3362S.
- ↑ "Structural basis of the activation of a metabotropic GABA receptor". Nature 584 (7820): 298–303. August 2020. doi:10.1038/s41586-020-2408-4. PMID 32555460. Bibcode: 2020Natur.584..298S.
- ↑ "Structures of metabotropic GABAB receptor". Nature 584 (7820): 310–314. June 2020. doi:10.1038/s41586-020-2469-4. PMID 32580208. Bibcode: 2020Natur.584..310P.
- ↑ "B receptor". Cell Research 30 (7): 564–573. June 2020. doi:10.1038/s41422-020-0350-5. PMID 32494023.
- ↑ "B receptor in an inactive state". Nature 584 (7820): 304–309. June 2020. doi:10.1038/s41586-020-2452-0. PMID 32581365.
- ↑ "Structural Basis for Activation of the Heterodimeric GABAB Receptor". Journal of Molecular Biology 432 (22): 5966–5984. November 2020. doi:10.1016/j.jmb.2020.09.023. PMID 33058878. https://pubmed.ncbi.nlm.nih.gov/33058878/.
- ↑ "Structural basis of GABAB receptor-Gi protein coupling". Nature 594 (7864): 594–598. June 2021. doi:10.1038/s41586-021-03507-1. PMID 33911284. Bibcode: 2021Natur.594..594S.
- ↑ "Positive allosteric modulation of native and recombinant gamma-aminobutyric acid(B) receptors by 2,6-Di-tert-butyl-4-(3-hydroxy-2,2-dimethyl-propyl)-phenol (CGP7930) and its aldehyde analog CGP13501". Molecular Pharmacology 60 (5): 963–71. November 2001. doi:10.1124/mol.60.5.963. PMID 11641424. http://molpharm.aspetjournals.org/cgi/content/abstract/60/5/963.
- ↑ "CGP7930: a positive allosteric modulator of the GABAB receptor". CNS Drug Reviews 13 (3): 308–16. 2007. doi:10.1111/j.1527-3458.2007.00021.x. PMID 17894647.
- ↑ "Positive modulation of GABA(B) receptors decreased nicotine self-administration and counteracted nicotine-induced enhancement of brain reward function in rats". The Journal of Pharmacology and Experimental Therapeutics 326 (1): 306–14. July 2008. doi:10.1124/jpet.108.139204. PMID 18445779.
- ↑ "N,N'-Dicyclopentyl-2-methylsulfanyl-5-nitro-pyrimidine-4,6-diamine (GS39783) and structurally related compounds: novel allosteric enhancers of gamma-aminobutyric acidB receptor function". The Journal of Pharmacology and Experimental Therapeutics 307 (1): 322–30. October 2003. doi:10.1124/jpet.103.053074. PMID 12954816.
- ↑ "Homotaurine: a GABAB antagonist in guinea-pig ileum". British Journal of Pharmacology 79 (4): 855–62. August 1983. doi:10.1111/j.1476-5381.1983.tb10529.x. PMID 6652358.
- ↑ "Interactions of ginsenosides with ligand-bindings of GABA(A) and GABA(B) receptors". General Pharmacology 25 (1): 193–9. January 1994. doi:10.1016/0306-3623(94)90032-9. PMID 8026706.
- ↑ "SGS742: the first GABA(B) receptor antagonist in clinical trials". Biochemical Pharmacology 68 (8): 1479–87. October 2004. doi:10.1016/j.bcp.2004.07.030. PMID 15451390.
- ↑ "SGS-742 Novartis". Current Opinion in Investigational Drugs 6 (1): 108–13. January 2005. PMID 15675610.
External links
 |
