Biology:Kavalactone
Kavalactones are a class of lactone compounds found in kava roots and Alpinia zerumbet (shell ginger).[1] Kavalactones are under research for potential to have various psychotropic effects, including anxiolytic and sedative/hypnotic activities.
Enzyme inhibition
Kava extract has been shown in vitro to potentially inhibit a wide range of hepatic enzymes, suggesting a possible potential for interactions with many pharmaceuticals and herbal medications.[2] In human volunteers in vivo inhibition is currently limited to CYP1A2,[3] and CYP2E1[4] through use of probe drugs to measure inhibition.
Research
Several preliminary studies are assessing potential effects of kava, including its anxiolytic actions[5] and hepatotoxicity, but the role specifically of kavalactones among many other kava compounds for these effects remains under study.[6][7]
The major kavalactones (except for desmethoxyyangonin) have been shown to potentiate the activity of GABAA receptors, which may underlie the anxiolytic and sedative properties of kava. Further, inhibition of the reuptake of norepinephrine and dopamine, binding to the CB1 receptor,[8] inhibition of voltage-gated sodium and calcium channels, and monoamine oxidase B reversible inhibition are additional pharmacological actions that have been reported for kavalactones.[9]
Kavalactone type compounds may help protect against high glucose induced cell damage.[1]
Toxicity
Several kavalactones (e.g. methysticin and yangonin) affect a group of enzymes involved in metabolism, called CYP1A1. Hepatotoxicity occurred in a small portion of previously healthy kava users,[6][10] particularly from extracts, as opposed to whole root powders.
Compounds
At least 18 different kavalactones have been identified to date, with methysticin being the first identified.[11] Multiple analogues, such as ethysticin, have also been isolated.[12] Some consist of a substituted α-pyrone as the lactone while others are partially saturated.
The average elimination half-life of kavalactones typically present in kava root is 9 hr.[13]
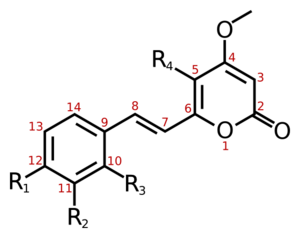
| Name | Structure | R1 | R2 | R3 | R4 |
|---|---|---|---|---|---|
| Yangonin | 1 | -OCH3 | -H | -H | -H |
| 10-methoxyyangonin | 1 | -OCH3 | -H | -OCH3 | -H |
| 11-methoxyyangonin | 1 | -OCH3 | -OCH3 | -H | -H |
| 11-hydroxyyangonin | 1 | -OCH3 | -OH | -H | -H |
| Desmethoxyyangonin | 1 | -H | -H | -H | -H |
| 11-methoxy-12-hydroxydehydrokavain | 1 | -OH | -OCH3 | -H | -H |
| 7,8-dihydroyangonin | 2 | -OCH3 | -H | -H | -H |
| Kavain | 3 | -H | -H | -H | -H |
| 5-hydroxykavain | 3 | -H | -H | -H | -OH |
| 5,6-dihydroyangonin | 3 | -OCH3 | -H | -H | -H |
| 7,8-dihydrokavain | 4 | -H | -H | -H | -H |
| 5,6,7,8-tetrahydroyangonin | 4 | -OCH3 | -H | -H | -H |
| 5,6-dehydromethysticin | 5 | -O-CH2-O- | -H | -H | |
| Methysticin | 7 | -O-CH2-O- | -H | -H | |
| 7,8-dihydromethysticin | 8 | -O-CH2-O- | -H | -H | |
Biosynthesis
The kavalactone biosynthetic pathway in Piper methysticum was described in 2019.[14]
See also
References
- ↑ 1.0 1.1 You, Hualin; He, Min; Pan, Di; Fang, Guanqin; Chen, Yan; Zhang, Xu; Shen, Xiangchun; Zhang, Nenling (2022). "Kavalactones isolated from Alpinia zerumbet (Pers.) Burtt. Et Smith with protective effects against human umbilical vein endothelial cell damage induced by high glucose". Natural Product Research 36 (22): 5740–5746. doi:10.1080/14786419.2021.2023866. PMID 34989299. https://www.tandfonline.com/doi/abs/10.1080/14786419.2021.2023866?journalCode=gnpl20.
- ↑ James M. Mathews; Amy S. Etheridge; Sherry R. Black (2002). "Inhibition of Human Cytochrome P450 Activities by Kava Extract and Kavalactones". Drug Metabolism and Disposition 30 (11): 1153–1157. doi:10.1124/dmd.30.11.1153. PMID 12386118. http://dmd.aspetjournals.org/content/30/11/1153.short.
- ↑ Russmann, S; Lauterburg, B; Barguil, Y; Choblet, E; Cabalion, P; Rentsch, K; Wenk, M (2005). "Traditional aqueous kava extracts inhibit cytochrome P450 1A2 in humans: Protective effect against environmental carcinogens?" (in en). Clinical Pharmacology & Therapeutics 77 (5): 453–454. doi:10.1016/j.clpt.2005.01.021. PMID 15900292. http://doi.wiley.com/10.1016/j.clpt.2005.01.021.
- ↑ Gurley, B; Gardner, S; Hubbard, M; Williams, D; Gentry, W; Khan, I; Shah, A (2005). "In vivo effects of goldenseal, kava kava, black cohosh, and valerian on human cytochrome P450 1A2, 2D6, 2E1, and 3A4/5 phenotypes" (in en). Clinical Pharmacology & Therapeutics 77 (5): 415–426. doi:10.1016/j.clpt.2005.01.009. PMID 15900287.
- ↑ Sarris, Jerome; LaPorte, Emma; Schweitzer, Isaac (2011-01-01). "Kava: A Comprehensive Review of Efficacy, Safety, and Psychopharmacology" (in en). Australian & New Zealand Journal of Psychiatry 45 (1): 27–35. doi:10.3109/00048674.2010.522554. PMID 21073405.
- ↑ 6.0 6.1 Teschke, R; Lebot, V (2011). "Proposal for a kava quality standardization code". Food and Chemical Toxicology 49 (10): 2503–16. doi:10.1016/j.fct.2011.06.075. PMID 21756963.
- ↑ Wang, J; Qu, W; Bittenbender, H. C.; Li, Q. X. (2013). "Kavalactone content and chemotype of kava beverages prepared from roots and rhizomes of Isa and Mahakea varieties and extraction efficiency of kavalactones using different solvents". Journal of Food Science and Technology 52 (2): 1164–1169. doi:10.1007/s13197-013-1047-2. PMID 25694734.
- ↑ "Kavalactones and the endocannabinoid system: the plant-derived yangonin is a novel CB₁ receptor ligand". Pharmacol. Res. 66 (2): 163–9. 2012. doi:10.1016/j.phrs.2012.04.003. PMID 22525682.
- ↑ "Therapeutic potential of kava in the treatment of anxiety disorders". CNS Drugs 16 (11): 731–43. 2002. doi:10.2165/00023210-200216110-00002. PMID 12383029.
- ↑ Teschke, R; Qiu, S. X.; Xuan, T. D.; Lebot, V (2011). "Kava and kava hepatotoxicity: Requirements for novel experimental, ethnobotanical and clinical studies based on a review of the evidence". Phytotherapy Research 25 (9): 1263–74. doi:10.1002/ptr.3464. PMID 21442674.
- ↑ Naumov, P.; Dragull, K.; Yoshioka, M.; Tang, C.-S.; Ng, S. W. (2008). "Structural Characterization of Genuine (-)-Pipermethystine, (-)-Epoxypipermethystine, (+)-Dihydromethysticin and Yangonin from the Kava Plant (Piper methysticum)". Natural Product Communications 3 (8): 1333–1336. doi:10.1177/1934578X0800300819. http://www.naturalproduct.us/.
- ↑ Shulgin, A. (1973). "The narcotic pepper - the chemistry and pharmacology of Piper methysticum and related species". Bulletin on Narcotics (2): 59–74. http://www.unodc.org/unodc/en/data-and-analysis/bulletin/bulletin_1973-01-01_2_page008.html.
- ↑ "Kava (Piper methysticum): Pharmacodynamics/Kinetics". Sigma-Aldrich Co. LLC. 2010. http://www.sigmaaldrich.com/life-science/nutrition-research/learning-center/plant-profiler/piper-methysticum.html.
- ↑ Pluskal, Tomáš; Torrens-Spence, Michael P.; Fallon, Timothy R.; De Abreu, Andrea; Shi, Cindy H.; Weng, Jing-Ke (2019-07-22). "The biosynthetic origin of psychoactive kavalactones in kava". Nature Plants (Springer Science and Business Media LLC) 5 (8): 867–878. doi:10.1038/s41477-019-0474-0. ISSN 2055-0278. PMID 31332312.
External links
- "NIH Kava Chemistry & Toxicology, Executive Summary". http://www.erowid.org/plants/kava/kava_chemistry2.shtml.
- The great kava boom: how Fiji's beloved psychoactive brew is going global The Guardian, 2020
 |
