Chemistry:Thiamylal
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | Thiamylal, Thioseconal, Surital |
| AHFS/Drugs.com | International Drug Names |
| ATCvet code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Metabolism | Hepatic |
| Elimination half-life | 14.3 h (cats) |
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank |
|
| ChemSpider |
|
| UNII | |
| KEGG |
|
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
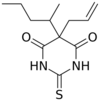
| Formula | C12H18N2O2S |
| Molar mass | 254.35 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Thiamylal (Surital) is a barbiturate derivative invented in the 1950s. It has sedative, anticonvulsant, and hypnotic effects, and is used as a strong but short acting sedative. Thiamylal is still in current use, primarily for induction in surgical anaesthesia[1] or as an anticonvulsant to counteract side effects from other anaesthetics.[2] It is the thiobarbiturate analogue of secobarbital.
References
- ↑ "Deep sedation with methohexital or thiamylal with midazolam for invasive procedures in children with acute lymphoblastic leukemia". Acta Paediatrica Taiwanica = Taiwan Er Ke Yi Xue Hui Za Zhi 46 (5): 294–300. 2005. PMID 16640004.
- ↑ "Seizure after local anesthesia for nasopharyngeal angiofibroma". The Kaohsiung Journal of Medical Sciences 23 (2): 97–100. February 2007. doi:10.1016/S1607-551X(09)70383-3. PMID 17339174.
| Alcohols | |
|---|---|
| Barbiturates |
|
| Benzodiazepines |
|
| Carbamates | |
| Flavonoids | |
| Imidazoles | |
| Kava constituents | |
| Monoureides | |
| Neuroactive steroids |
|
| Nonbenzodiazepines | |
| Phenols | |
| Piperidinediones | |
| Pyrazolopyridines | |
| Quinazolinones | |
| Volatiles/gases |
|
| Others/unsorted |
|
| CAR |
|
|---|---|
| PXR |
|
| Inhalational | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Injection |
| ||||||||||||
| |||||||||||||
 | 0.00      (0 votes) (0 votes) |
