Chemistry:Emylcamate
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
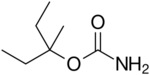
| Formula | C7H15NO2 |
| Molar mass | 145.202 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Emylcamate (marketed as Striatran by Merck) is an anxiolytic and muscle relaxant. It was patented in the US in 1961 (US Patent 2,972,564) and advertised for the treatment of anxiety and tension. It was claimed to be superior to meprobamate, which would eventually replace emylcamate.
A study of the drug's effects in mice was done in 1959. It concluded that at 50 mg/kg emylcamate gave a 63% decrease in motor activity compared with meprobamate's 32% decrease, a doubling in effective potency. The therapeutic index in mice was also established:
| Meprobamate | Emylcamate | Effect |
|---|---|---|
| 175 | 123 | ED50 (mg/kg) |
| 600 | 550 | LD50 (mg/kg) |
| 3.4 | 4.4 | Therapeutic index |
Emylcamate also has a faster intra-parenteral onset than meprobamate, 3 minutes compared with 35. [1]
References
- ↑ "Emylcamate, A Potent Tranquillizing Relaxant". Journal of Medicinal and Pharmaceutical Chemistry 1 (5): 443–457. 1959. doi:10.1021/jm50006a003.
Further reading
- "Olhando para trás: um novo caminho possível para a descoberta de drogas em psicofarmacologia." (in pt). Revista de Psiquiatria do Rio Grande do Sul 26 (2): 196–203. August 2004. doi:10.1590/S0101-81082004000200009.
External links
- "Emylcamate". The Comparative Toxicogenomics Database. http://ctdbase.org/detail.go?type=chem&acc=C100111.
- "Emylcamate" (in fr). BIAM. http://www.biam2.org/www/Sub2770.html.
 |

