Chemistry:Methohexital
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Brevital Sodium |
| Other names | Methohexitone |
| AHFS/Drugs.com | Consumer Drug Information |
| License data |
|
| Pregnancy category |
|
| Routes of administration | Intravenous, rectal |
| Drug class | Barbiturate |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | I.V. ~100% Rectal ~17% |
| Metabolism | Liver |
| Elimination half-life | 5.6 ± 2.7 minutes |
| Excretion | excreted in feces |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C14H18N2O3 |
| Molar mass | 262.309 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Methohexital or methohexitone (marketed under the brand names Brevital and Brietal) is a drug which is a barbiturate derivative. It is classified as short-acting, and has a rapid onset of action.[2] It is similar in its effects to sodium thiopental, a drug with which it competed in the market for anesthetics.
Pharmacology
Methohexital binds to a distinct site which is associated with Cl− ionophores at GABAA receptors.[3] This increases the length of time which the Cl− ionopores are open, thus causing an inhibitory effect.
Metabolism of methohexital is primarily hepatic via demethylation and oxidation.[1] Side-chain oxidation is the primary means of metabolism involved in the termination of the drug's biological activity.
Indications
Methohexital is primarily used to induce anesthesia, and is generally provided as a sodium salt (i.e. methohexital sodium). It is only used in hospital or similar settings, under strict supervision.[1] It has been commonly used to induce deep sedation or general anesthesia for surgery and dental procedures. Unlike many other barbiturates, methohexital actually lowers the seizure threshold, a property that makes it particularly useful when anesthesia is provided for an electroconvulsive therapy (ECT).[4] Its rapid recovery rate with consciousness being gained within three to seven minutes after induction and full recovery within 30 minutes is a major advantage over other ECT barbiturates.[4]
Synthesis
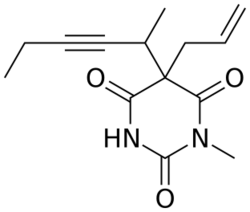
Methohexital can be synthesized in the classic manner of making barbituric acid derivatives, in particular by the reaction of malonic ester derivatives with derivatives of urea.[5] The resulting allyl-(1-methyl-2-pentynyl) malonic ester is synthesized by subsequent alkylation of the malonic ester itself, beginning with 2-bromo-3-hexyne, which gives (1-methyl-2-pentynyl)malonic ester, and then by allylbromide. In the final step, reaction of the disubstituted malonic ester with N-methylurea gives methohexital.
References
- ↑ 1.0 1.1 1.2 "Brevital Sodium". July 24, 2019. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=eccd8340-ead3-4363-8902-0c19d33aa2ac.
- ↑ "Methohexital". https://www.ncbi.nlm.nih.gov/mesh/68008723.
- ↑ Basic and Clinical Pharmacology (10th ed.). pp. 406–407.
- ↑ 4.0 4.1 "Methohexital anaesthesia in electroconvulsive therapy". South African Medical Journal 37: 870–1. August 1963. PMID 14045806.
- ↑ Doran WJ, "1,5,5-Trisubstituted barbituric acids", US patent 2872448, issued February 3, 1959, assigned to Eli Lily and Company (U.S. Patent 2,872,448)
External links
- "Methohexital". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/rn/151-83-7.
- "Methohexital sodium". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/methohexital%20sodium.
 |

