Chemistry:Progesterone dioxime
From HandWiki
Short description: Chemical compound
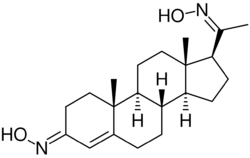 | |
| Clinical data | |
|---|---|
| Other names | Progesterone 3,20-dioxime; P4-3,20-DO; Pregn-4-ene-3,20-dione 3,20-dioxime; 3,20-Di(hydroxyimino)pregn-4-en-3-one |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| Chemical and physical data | |
| Formula | C21H32N2O2 |
| Molar mass | 344.499 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 238–242 °C (460–468 °F) (recrystallized from alcohol) |
| |
| |
Progesterone dioxime, or progesterone 3,20-dioxime (P4-3,20-DO), also known as 3,20-di(hydroxyimino)pregn-4-en-3-one, is a progesterone derivative which was never marketed.[1] It is a progestogen oxime – specifically, the C3 and C20 dioxime of the progestogen progesterone.[1] Progesterone C3 and C20 oxime conjugates have been found to be water-soluble prodrugs of progesterone and pregnane neurosteroids.[2][3][4][5]
See also
References
- ↑ 1.0 1.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 1024–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA1024.
- ↑ "Development and screening of water-soluble analogues of progesterone and allopregnanolone in models of brain injury". Journal of Medicinal Chemistry 52 (19): 6012–6023. October 2009. doi:10.1021/jm900712n. PMID 19791804.
- ↑ "Water-soluble progesterone analogues are effective, injectable treatments in animal models of traumatic brain injury". ACS Medicinal Chemistry Letters 3 (5): 362–366. May 2012. doi:10.1021/ml200303r. PMID 24900479.
- ↑ "Evaluating the neurotherapeutic potential of a water-soluble progesterone analog after traumatic brain injury in rats". Neuropharmacology 109: 148–158. October 2016. doi:10.1016/j.neuropharm.2016.05.017. PMID 27267687.
- ↑ "Effects of 3-hydrazone modification on the metabolism and protein binding of progesterone". International Journal of Pharmaceutics 65 (1–2): 109–114. 1990. doi:10.1016/0378-5173(90)90015-V. ISSN 0378-5173.
 |
