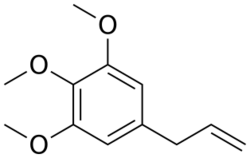
Chemistry:Elemicin
 | |
 | |
| Clinical data | |
|---|---|
| Dependence liability | None |
| Addiction liability | Low / None |
| Routes of administration | Oral |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C12H16O3 |
| Molar mass | 208.257 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Elemicin is a phenylpropene, a natural organic compound, and is a constituent of several plant species' essential oils.[1][2]
Natural occurrence
Elemicin is a constituent of the oleoresin and the essential oil of Canarium luzonicum (also referred to as elemi). Elemicin is named after this tree. One study found it to compose 2.4% of the fresh essential oil.[1] Elemicin is also present in the oils of the spices nutmeg and mace, with it composing 2.4% and 10.5% of those oils respectively.[2] Structurally, elemicin is similar to myristicin, differing only by myristicin's methyl group that joins the two oxygen atoms that make up its dioxymethy moiety, with both constituents being found in nutmeg and mace.
Isolation
Elemicin was first isolated from elemi oil using vacuum distillation. Specifically, the substance was collected between 162-165 °C at a reduced pressure of 10 torr.[3][4]
Preparation
Elemicin has been synthesized from syringol and allyl bromide using Williamson ether synthesis and Claisen rearrangement.[5][6] The electrophilic aromatic substitution entering the para-position was made possible by secondary Cope rearrangement.[7] This is due to syringol's allyl aromatic ether being blocked by ethers in both ortho-positions. When blocked the allyl group migrates to the para-position, in this case with yields above 85%.[8]
Uses
Elemicin has been used to synthesize the proto-alkaloid mescaline.[9]
Pharmacology
Raw nutmeg causes anticholinergic-like effects, which are attributed to elemicin and myristicin.[10][11] Elemicin inhibits Stearoyl-CoA Desaturase 1 (SCD1) by metabolic activation. Elemicin is one of the main components in aromatic food and has antimicrobial, antioxidant, and antiviral activities. Elemicin possesses genotoxicity and carcinogenicity.[12][13] Excess consumption of raw nutmeg results in delirium and disorientation.[14]
Elemicin's psychoactivity is still a point of research, however some research suggests it may act like an agonist of the 5-HT2A receptors, similar to many psychedelics.[15] However, this is controversial as the psychoactive effects of elemicin and plants it is found in, such as nutmeg, seem to cause more deliriant-like effects than psychedelic ones.[16]
See also
References
- ↑ 1.0 1.1 "The Composition of Manila Elemi Oil". Flavour and Fragrance Journal 8: 35–37. 1993. doi:10.1002/ffj.2730080107.
- ↑ 2.0 2.1 Chemistry of Spices. Calicut, Kerala, India: Biddles Ltd.. 2008. pp. 165–188 [170]. ISBN 9781845934057. https://books.google.com/books?id=5WY08iuJyawC&q=Chemistry+of+Spices.
- ↑ "Constituents of Essential Oils. Elemicin, a High-boiling Constituent of Elemi Oil, and the Displacement of Alkyloxygroups in the Benzene Nucleus by Hydrogen". Journal of the Chemical Society, Abstracts 94 (A493): 557–558. 1908. doi:10.1039/CA9089400493. https://books.google.com/books?id=JGJFAQAAIAAJ&q=Constituents+of+Essential+Oils.+Elemicin%2C+a+High-boiling+Constituent+of+Elemi+Oil%2C+and+the+Displacement+of+Alkyloxygroups+in+the+Benzene+Nucleus+by+Hydrogen&pg=PA557.
- ↑ "Zur Kenntnis der Bestandteile der ätherischen Öle. (Über das Elemicin, einen hochsiedenden Bestandteil des Elemiöls, und über Ersetzung von Alkyloxy-gruppen am Benzolkern durch Wasserstoff.)". Berichte der Deutschen Chemischen Gesellschaft 41 (2): 1768–1775. 1908. doi:10.1002/cber.19080410240. https://zenodo.org/record/1426299.
- ↑ "Die Synthese des Elemicins und Isoelemicins". Justus Liebigs Annalen der Chemie 414 (2): 250–255. 1918. doi:10.1002/jlac.19184140213. https://zenodo.org/record/1427645.
- ↑ "Synthesis of Elemicin and of isoElemicin". Journal of the Chemical Society, Abstracts 114: i428. 1918. doi:10.1039/CA9181400421.
- ↑ Named Organic Reactions, 2nd Edition. Wolfsburg, Germany: John Wiley & Sons, Ltd.. 2005. pp. 58–60 [59]. ISBN 978-0470010402. https://books.google.com/books?id=-BEdbO-08WMC&q=Named+Organic+Reactions,+2nd+Edition+2005.
- ↑ "The Claisen Rearrangement". Organic Reactions. II. New York: John Wiley & Sons, Inc.. 1944. pp. 2–44 [44]. doi:10.1002/0471264180.or002.01. ISBN 978-0471264187. http://library.sciencemadness.org/library/books/organic_reactions_v2.pdf.
- ↑ "Über β-[Oxyphenyl]-äthylamine und ihre Umwandlungen, I. Mitteil.: Synthese des Mezcalins". Berichte der Deutschen Chemischen Gesellschaft (A and B Series) 67 (4): 696–708. 1934. doi:10.1002/cber.19340670430.
- ↑ "Acute nutmeg poisoning". European Journal of Emergency Medicine 11 (4): 240–241. August 2004. doi:10.1097/01.mej.0000127649.69328.a5. PMID 15249817.
- ↑ "The chemistry and psychopharmacology of nutmeg and of several related phenylisopropylamines" (pdf). Psychopharmacology Bulletin 4 (3): 13. December 1967. PMID 5615546. http://bitnest.ca/external.php?id=%250E%253D9%250F%2524G%252F%2518B%255B%255B4%2522.FXQ%255CO%2500TK. Retrieved 2015-09-02.
- ↑ "Elemicin" (in en). https://www.chemsrc.com/en/cas/487-11-6_954455.html.
- ↑ "Elemicin | Stearoyl-CoA Desaturase (SCD) Inhibitor | MedChemExpress". https://www.medchemexpress.com/elemicin.html.
- ↑ "elemicin", The Free Dictionary, https://medical-dictionary.thefreedictionary.com/elemicin, retrieved 2022-10-28
- ↑ Evaluation of 5-HT2A Receptor Agonistic Property of Elemicin
- ↑ "Nutmeg and psychosis". Schizophrenia Research 60 (1): 95–96. March 2003. doi:10.1016/s0920-9964(02)00204-9. PMID 12505144.
 |

