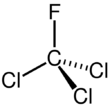
Chemistry:Trichlorofluoromethane
|
| |||
| Names | |||
|---|---|---|---|
| Preferred IUPAC name
Trichloro(fluoro)methane | |||
| Other names
Trichlorofluoromethane
Fluorotrichloromethane Fluorochloroform Freon 11 CFC 11 R 11 Arcton 9 Freon 11A Freon 11B Freon HE Freon MF | |||
| Identifiers | |||
3D model (JSmol)
|
|||
| ChEBI | |||
| ChEMBL | |||
| ChemSpider | |||
| EC Number |
| ||
PubChem CID
|
|||
| RTECS number |
| ||
| UNII | |||
| |||
| |||
| Properties | |||
| CCl3F | |||
| Molar mass | 137.36 g·mol−1 | ||
| Appearance | Colorless liquid/gas | ||
| Odor | nearly odorless[1] | ||
| Density | 1.494 g/cm3 | ||
| Melting point | −110.48 °C (−166.86 °F; 162.67 K) | ||
| Boiling point | 23.77 °C (74.79 °F; 296.92 K) | ||
| 1.1 g/L (at 20 °C) | |||
| log P | 2.53 | ||
| Vapor pressure | 89 kPa at 20 °C 131 kPa at 30 °C | ||
| Thermal conductivity | 0.0079 W m−1 K−1 (gas at 300 K, ignoring pressure dependence)[2][verification needed] | ||
| Hazards | |||
| Safety data sheet | ICSC 0047 | ||
| GHS pictograms | 
| ||
| GHS Signal word | warning | ||
| Flash point | Non-flammable | ||
| Lethal dose or concentration (LD, LC): | |||
LCLo (lowest published)
|
26,200 ppm (rat, 4 hr) 100,000 ppm (rat, 20 min) 100,000 ppm (rat, 2 hr)[3] | ||
| NIOSH (US health exposure limits): | |||
PEL (Permissible)
|
TWA 1000 ppm (5600 mg/m3)[1] | ||
REL (Recommended)
|
C 1000 ppm (5600 mg/m3)[1] | ||
IDLH (Immediate danger)
|
2000 ppm[1] | ||
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |||
| Infobox references | |||
Trichlorofluoromethane, also called freon-11, CFC-11, or R-11, is a chlorofluorocarbon (CFC). It is a colorless, faintly ethereal, and sweetish-smelling liquid that boils around room temperature.[5] CFC-11 is a Class 1 ozone-depleting substance which damages Earth's protective stratospheric ozone layer.[6]
Historical use
Trichlorofluoromethane was first widely used as a refrigerant. Because of its high boiling point compared to most refrigerants, it can be used in systems with a low operating pressure, making the mechanical design of such systems less demanding than that of higher-pressure refrigerants R-12 or R-22.
Trichlorofluoromethane is used as a reference compound for fluorine-19 NMR studies.
Trichlorofluoromethane was formerly used in the drinking bird novelty, largely because it has a boiling point of 23.77 °C (74.79 °F). The replacement, dichloromethane, boiling point 39.6 °C (103.3 °F), requires a higher ambient temperature to work.
Prior to the knowledge of the ozone depletion potential of chlorine in refrigerants and other possible harmful effects on the environment, trichlorofluoromethane was sometimes used as a cleaning/rinsing agent for low-pressure systems.[7]
Production
Trichlorofluoromethane can be obtained by reacting carbon tetrachloride with hydrogen fluoride at 435 °C and 70 atm, producing a mixture of trichlorofluoromethane, tetrafluoromethane and dichlorodifluoromethane in a ratio of 77:18:5. The reaction can also be carried out in the presence of antimony(III) chloride or antimony(V) chloride:[8]
- [math]\ce{ CCl4 + HF -> CCl3F + CF4 + CCl2F2 }[/math]
Trichlorofluoromethane is also formed as one of the byproducts when graphite reacts with chlorine and hydrogen fluoride at 500 °C.[8]
Sodium hexafluorosilicate under pressure at 270 °C, titanium(IV) fluoride, chlorine trifluoride, cobalt(III) fluoride, iodine pentafluoride, and bromine trifluoride are also suitable fluorinating agents for carbon tetrachloride.[8][9]
- [math]\ce{ CCl4 + Na2SiF6 -> CCl3F + CCl2F2 + CCl3F + NaCl + SiF4 }[/math]
- [math]\ce{ CCl4 + BrF3 -> BrF + CCl2F2 + CCl3F }[/math]
Trichlorofluoromethane was included in the production moratorium in the Montreal Protocol of 1987. It is assigned an ozone depletion potential of 1.0, and U.S. production was ended on January 1, 1996.[6]
Regulatory challenges
In 2018, the atmospheric concentration of CFC-11 was noted by researchers to be declining more slowly than expected,[10][11] and it subsequently emerged that it remains in widespread use as a blowing agent for polyurethane foam insulation in the construction industry of China .[12] In 2021, researchers announced that emissions declined by 20,000 U.S. tons from 2018 to 2019, which mostly reversed the previous spike in emissions.[13] In 2022, the European Commission announced an updated regulation that mandates the recovery and prevention of emissions of CFC-11 blowing agents from foam insulation in demolition waste, which is still emitted at significant scale.[14]
Dangers
R11, like most chlorofluorocarbons, forms phosgene gas when exposed to a naked flame.[15]
Gallery
CFC-11 measured by the Advanced Global Atmospheric Gases Experiment (AGAGE) in the lower atmosphere (troposphere) at stations around the world. Abundances are given as pollution free monthly mean mole fractions in parts-per-trillion.
See also
References
- ↑ 1.0 1.1 1.2 1.3 NIOSH Pocket Guide to Chemical Hazards. "#0290". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0290.html.
- ↑ Touloukian, Y.S., Liley, P.E., and Saxena, S.C. Thermophysical properties of matter - the TPRC data series. Volume 3. Thermal conductivity - nonmetallic liquids and gases. Data book. 1970.
- ↑ "Fluorotrichloromethane". Immediately Dangerous to Life and Health Concentrations (IDLH). National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/idlh/75694.html.
- ↑ Record in the GESTIS Substance Database of the Institute for Occupational Safety and Health
- ↑ Siegemund, Günter; Schwertfeger, Werner; Feiring, Andrew; Smart, Bruce; Behr, Fred; Vogel, Herward; McKusick, Blaine (2002). "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a11_349.
- ↑ 6.0 6.1 "International Treaties and Cooperation about the Protection of the Stratospheric Ozone Layer". U.S. Environmental Protection Agency. 15 July 2015. https://www.epa.gov/ozone-layer-protection/international-treaties-and-cooperation-about-protection-stratospheric-ozone. Retrieved 2021-02-14.
- ↑ "R-10 ,R-11 ,R-12 GASES - ملتقى التبريد والتكييف HVACafe" (in ar-AR). ملتقى التبريد والتكييف HVACafe. 2017-05-25. https://hvacafe.com/r-10-r-11-r-12-gases/.
- ↑ 8.0 8.1 8.2 Alan R. Katritzky, Thomas L. Gilchrist, Otto Meth-Cohn, Charles Wayne Rees (1995), [[1], p. 220, at Google Books Comprehensive Organic Functional Group Transformations], Elsevier, pp. 220, ISBN 978-0-08-042704-1, [2], p. 220, at Google Books
- ↑ A. A. Banks, H. J. Emeléus u. a.: 443. The reaction of bromine trifluoride and iodine pentafluoride with carbon tetrachloride, tetrabromide, and tetraiodide and with tetraiodoethylene. In: J. Chem. Soc., 1948, S. 2188, doi:10.1039/JR9480002188.
- ↑ Montzka, S.A.; Dutton, G.S.; Yu, P. (2018). "An unexpected and persistent increase in global emissions of ozone-depleting CFC-11". Nature (Springer Nature) 557 (7705): 413–417. doi:10.1038/s41586-018-0106-2. PMID 29769666. Bibcode: 2018Natur.557..413M. https://www.nature.com/articles/s41586-018-0106-2.
- ↑ Johnson, Scott (5 May 2018). "It seems someone is producing a banned ozone-depleting chemical again". https://arstechnica.com/science/2018/05/it-seems-someone-is-producing-a-banned-ozone-depleting-chemical-again/. "Decline of CFC-11 has slowed in recent years, pointing to a renewed source"
- ↑ McGrath, Matt (9 July 2018). "China 'home foam' gas key to ozone mystery". https://www.bbc.co.uk/news/science-environment-44738952.
- ↑ Jennifer Chu (2021-02-10). "Reductions in CFC-11 emissions put ozone recovery back on track". MIT News. https://news.mit.edu/2021/cfc-11-ozone-recovery-0210.
- ↑ "Proposal for a regulation of the european parliament and the council on substances that deplete the ozone layer". https://climate.ec.europa.eu/system/files/2022-04/ods_proposal_en.pdf.
- ↑ "False Alarms: The Legacy of Phosgene Gas". 4 January 2021. https://hvacrschool.com/phosgene-gas/.
External links
- CFC-11 NOAA/ESRL Global measurements
- Public health goal for trichlorofluoromethane in drinking water
- Names at webbook.nist.gov
- Data sheet at speclab.com
- International Chemical Safety Card 0047
- NIOSH Pocket Guide to Chemical Hazards. "#0290". National Institute for Occupational Safety and Health (NIOSH). https://www.cdc.gov/niosh/npg/npgd0290.html.
- Phase change data at webbook.nist.gov
- Thermochemistry data at chemnet.ru
- ChemSub Online: Trichlorofluoromethane - CFC-11
- materialsproject.org
 |