Chemistry:Propacetamol
From HandWiki
Short description: Chemical compound
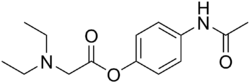 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | IV[1][2] |
| ATC code | |
| Pharmacokinetic data | |
| Elimination half-life | 2.4 hours [1] |
| Excretion | Renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C14H20N2O3 |
| Molar mass | 264.325 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Propacetamol is a prodrug form of paracetamol which is formed from esterification of paracetamol, and the carboxylic acid diethylglycine. This has the advantage of making it more water-soluble. It is used in post-operative care and is delivered by I.V.[2] It is given if the patient is unable to take oral or rectally delivered paracetamol and nonsteroidal anti-inflammatory drugs (NSAIDs) are contraindicated. The onset of analgesia from propacetamol is more rapid than paracetamol given orally.[3] 2 grams of propacetamol are equivalent to 1g of paracetamol.[4]
See also
References
- ↑ 1.0 1.1 "Plasma and cerebrospinal fluid concentrations of paracetamol after a single intravenous dose of propacetamol". British Journal of Clinical Pharmacology 34 (1): 79–81. 1992. doi:10.1111/j.1365-2125.1992.tb04112.x. PMID 1633071.
- ↑ 2.0 2.1 "Comparative effect of intraoperative propacetamol versus placebo on morphine consumption after elective reduction mammoplasty under remifentanil-based anesthesia: a randomized control trial [ISRCTN71723173]". BMC Anesthesiology 4 (1): 6. 2004. doi:10.1186/1471-2253-4-6. PMID 15367329.
- ↑ "Onset of acetaminophen analgesia: comparison of oral and intravenous routes after third molar surgery". British Journal of Anaesthesiology 94 (5): 642–648. 2005. doi:10.1093/bja/aei109. PMID 15790675.
- ↑ "Bioequivalence study comparing a new paracetamol solution for injection and propacetamol after single intravenous infusion in healthy subjects". International Journal of Clinical Pharmacology and Therapeutics 42 (1): 50–57. 2004. doi:10.5414/cpp42050. PMID 14756388.
 |

