Chemistry:Pentagestrone acetate
 | |
| Clinical data | |
|---|---|
| Trade names | Gestovis, Gestovister |
| Other names | PGA; Gestovis; 17α-Acetoxyprogesterone 3-cyclopentyl enol ether |
| Routes of administration | By mouth |
| Drug class | Progestogen; Progestin; Progestogen ether; Progestogen ester |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
| Formula | C28H40O4 |
| Molar mass | 440.624 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
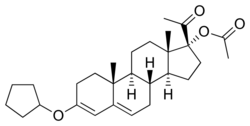
Pentagestrone acetate (PGA), sold under the brand names Gestovis and Gestovister, is a progestin which was described in the literature in 1960 and was introduced by Vister in Italy in 1961.[1][2][3] It is the 3-cyclopentyl enol ether of 17α-hydroxyprogesterone acetate.[4] PGA, along with quingestrone (the 3-cyclopentyl enol ether of progesterone), is said to have very similar properties to those of dydrogesterone, a pure progestogen and close analogue of progesterone.[5]
PGA is orally active, was provided in 10 and 20 mg capsules, and has been used to treat habitual abortion and menstrual disorders at a dosage of 10 to 20 mg/day.[6] It has been said to have equivalent potency to intramuscular progesterone.[6] The combination of 20 mg/day PGA and 100 μg/day mestranol is an effective ovulation inhibitor in women.[7][8] The effective dosage of PGA in the menstrual delay test has been studied.[9]
Chemistry
PGA, also known as 17α-acetoxyprogesterone 3-cyclopentyl enol ether, is a synthetic pregnane steroid and a derivative of progesterone and 17α-hydroxyprogesterone.[1]
See also
References
- ↑ 1.0 1.1 The Dictionary of Drugs: Chemical Data: Chemical Data, Structures and Bibliographies. Springer. 14 November 2014. pp. 943–. ISBN 978-1-4757-2085-3. https://books.google.com/books?id=0vXTBwAAQBAJ&pg=PA943.
- ↑ Drugs Available Abroad. Gale Research. 1991. ISBN 978-0-8103-7177-4. https://books.google.com/books?id=2x1tAAAAMAAJ.
- ↑ "Pentagestrone". Chemikalien und Drogen Teil A: N-Q. Springer-Verlag. 12 March 2013. pp. 508–. ISBN 978-3-642-65035-2. https://books.google.com/books?id=4TWnBgAAQBAJ&pg=PA508.
- ↑ "Designing Prodrugs and Bioprecursors". The Practice of Medicinal Chemistry. Academic Press. 2 May 2011. pp. 731–. ISBN 978-0-08-056877-5. https://books.google.com/books?id=Qmt1_DQkCpEC&pg=PA731.
- ↑ Revue générale des sciences pures et appliquées et bulletin de l'Association française pour l'avancement des sciences. Société d'édition d'enseignement supérieur. 1964. https://books.google.com/books?id=UuEkAQAAIAAJ. "[[...] Ercoli (1960) developed cyclopentyl enol ethers of progesterone (Luteovis) and acetoxy progesterone (Gestovis), which have almost exactly the same properties as dydrogesterone.]"
- ↑ 6.0 6.1 "Gestovis". South African Medical Journal 40 (5): 99. January 1966. ISSN 0256-9574. https://journals.co.za/content/m_samj/40/5/AJA20785135_36784. "Comopharm (Pty) Limited. on behalf of Vister Laboratories, announce the introduction of Gestovis, and supply the following information: Gestovis is a new highly effective oral progesterone derivative (cyclopentyl enol-ether of 17-alpha-acetoxy progesterone) for the treatment of threatened and habitual abortion and menstrual disturbances. Gestovis is efficacious orally at the same dose as parenterally administered progesterone. Dosage. 10 - 20 mg. daily or as necessary. Presentation. Available in capsules of 10 and 20 mg.".
- ↑ "The Inhibition of Ovulation". The Control of Fertility. Elsevier. 3 September 2013. pp. 221–. ISBN 978-1-4832-7088-3. https://books.google.com/books?id=ehQlBQAAQBAJ&pg=PA221.
- ↑ Current Medicine and Drugs. 1962. p. 32. https://books.google.com/books?id=auc1AQAAIAAJ. "Gestovis (3-cyclo-pentyl-enol ether of 17a acetoxyprogesterone) is a potent non-oestrogenic preparation but does not give very good cycle control. In doses of 20 mg daily from the fifth to the twenty-fifth day of the cycle it will inhibit ovulation."
- ↑ "Potencies of oral contraceptives". American Journal of Obstetrics and Gynecology 125 (8): 1029–1038. August 1976. doi:10.1016/0002-9378(76)90804-8. PMID 952300.
 |

