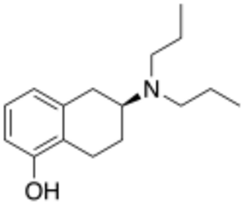
Chemistry:5-OH-DPAT
From HandWiki
Short description: Dopamine receptor agonist compound
| |
| Names | |
|---|---|
| Preferred IUPAC name
(6S)-6-(Dipropylamino)-5,6,7,8-tetrahydronaphthalen-1-ol | |
| Identifiers | |
3D model (JSmol)
|
|
| Abbreviations | 5-OH-DPAT |
| ChEMBL | |
| ChemSpider | |
| MeSH | 5-Hydroxy-2-N,N-dipropylaminotetralin |
PubChem CID
|
|
| |
| |
| Properties | |
| C16H25NO | |
| Molar mass | 247.382 g·mol−1 |
| log P | 3.55 |
| Acidity (pKa) | 10.543 |
| Basicity (pKb) | 3.454 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tracking categories (test):
5-OH-DPAT is a synthetic compound that acts as a dopamine receptor agonist with selectivity for the D2 receptor and D3 receptor subtypes.[1][2] Only the (S)-enantiomer is active as an agonist, with the (R)-enantiomer being a weak antagonist at D2 receptors.[3] Radiolabelled 11C-5-OH-DPAT is used as an agonist radioligand for mapping the distribution and function of D2 and D3 receptors in the brain,[4][5] and the drug is also being studied in the treatment of Parkinson's disease.[6][needs update]
See also
References
- ↑ "Structure-activity relationships of dopaminergic 5-hydroxy-2-aminotetralin derivatives with functionalized N-alkyl substituents". Journal of Medicinal Chemistry 29 (6): 912–7. June 1986. doi:10.1021/jm00156a007. PMID 3712381.
- ↑ "C3-methylated 5-hydroxy-2-(dipropylamino)tetralins: conformational and steric parameters of importance for central dopamine receptor activation". Journal of Medicinal Chemistry 30 (7): 1135–44. July 1987. doi:10.1021/jm00390a004. PMID 3599021.
- ↑ "(R)- and (S)-5-hydroxy-2-(dipropylamino)tetralin (5-OH DPAT): assessment of optical purities and dopaminergic activities". Chirality 2 (2): 90–5. 1990. doi:10.1002/chir.530020206. PMID 1976017.
- ↑ "In vitro and in vivo evaluation of the binding of the dopamine D2 receptor agonist (11)C-(R,S)-5-hydroxy-2-(di-n-propylamino)tetralin in rodents and nonhuman primate". Synapse (New York, N.Y.) 37 (1): 64–70. July 2000. doi:10.1002/(SICI)1098-2396(200007)37:1<64::AID-SYN7>3.0.CO;2-F. PMID 10842352.
- ↑ "(R,S)-2-(N-Propyl-N-1'-[11C]-propyl)amino-5-hydroxytetralin.". Molecular Imaging and Contrast Agent Database (MICAD) [Internet]. Bethesda (MD): National Center for Biotechnology Information (US). 2006. PMID 20641325.
- ↑ "The pharmacokinetics and pharmacological effect of (S)-5-OH-DPAT following controlled delivery with transdermal iontophoresis". Journal of Pharmaceutical Sciences 100 (7): 2996–3009. January 2011. doi:10.1002/jps.22492. PMID 21283984.
 |
