Chemistry:SKF-83,959
From HandWiki
Short description: Chemical compound
 | |
| Identifiers | |
|---|---|
| |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
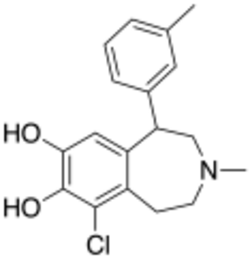
| Formula | C18H20ClNO2 |
| Molar mass | 317.81 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
SKF-83,959 is a synthetic benzazepine derivative used in scientific research which acts as an agonist at the D1–D2 dopamine receptor heteromer.[1] It behaves as a full agonist at the D1 protomer and a high-affinity partial agonist at the D2 protomer. It was further shown to act as an allosteric modulator of the sigma-1 receptor.[2] SKF-83,959 additionally inhibits sodium channels[3] as well as delayed rectifier potassium channels.[4] SKF-83,959 is a racemate that consists of the R-(+)- and S-(−)-enantiomers MCL-202 and MCL-201, respectively.
SKF-83,959 was described as a SNDRI.[5] The synthesis has been described:[sentence fragment][6]
References
- ↑ "D1-D2 dopamine receptor heterooligomers with unique pharmacology are coupled to rapid activation of Gq/11 in the striatum". Proc. Natl. Acad. Sci. U.S.A. 104 (2): 654–9. 2007. doi:10.1073/pnas.0604049104. PMID 17194762. Bibcode: 2007PNAS..104..654R.
- ↑ "SKF83959 is a potent allosteric modulator of sigma-1 receptor". Mol. Pharmacol. 83 (3): 577–86. 2013. doi:10.1124/mol.112.083840. PMID 23295385.
- ↑ "SKF83959 suppresses excitatory synaptic transmission in rat hippocampus via a dopamine receptor-independent mechanism". J. Neurosci. Res. 89 (8): 1259–66. 2011. doi:10.1002/jnr.22653. PMID 21538463.
- ↑ "Arylbenzazepines are potent modulators for the delayed rectifier K+ channel: a potential mechanism for their neuroprotective effects". PLOS ONE 4 (6): e5811. 2009. doi:10.1371/journal.pone.0005811. PMID 19503734. Bibcode: 2009PLoSO...4.5811C.
- ↑ "SKF83959 is a novel triple reuptake inhibitor that elicits anti-depressant activity". Acta Pharmacologica Sinica 34 (9): 1149–1155. September 2013. doi:10.1038/aps.2013.66. ISSN 1671-4083. PMID 23892272.
- ↑ "Dopaminergic activity of substituted 6-chloro-1-phenyl-2,3,4,5-tetrahydro-1H-3-benzazepines". Journal of Medicinal Chemistry 25 (4): 352–358. April 1982. doi:10.1021/jm00346a005. PMID 7069713.
Further reading
- "Dopamine D1 receptor signaling: Does GαQ-phospholipase C actually play a role?". J. Pharmacol. Exp. Ther. 351 (1): 9–17. 2014. doi:10.1124/jpet.114.214411. PMID 25052835.
- "SKF-83959 is not a highly-biased functionally selective D1 dopamine receptor ligand with activity at phospholipase C". Neuropharmacology 86: 145–54. 2014. doi:10.1016/j.neuropharm.2014.05.042. PMID 24929112.
 |

