Chemistry:Propylnorapomorphine
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Routes of administration | Oral |
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| Chemical and physical data | |
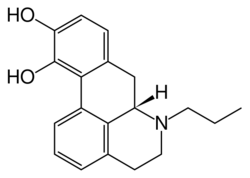
| Formula | C19H21NO2 |
| Molar mass | 295.382 g·mol−1 |
| 3D model (JSmol) | |
| Density | 1.2 g/cm3 |
| Boiling point | 446 to 536 °C (835 to 997 °F) [1] |
| |
N-n-Propylnorapomorphine (NPA) is an aporphine derivative dopamine agonist closely related to apomorphine.[2][3] In rodents it has been shown to produce hyperactivity, stereotypy, hypothermia, antinociception, and penile erection, among other effects.[4][5][6][7] Notably, its effects on locomotion are biphasic, with low doses producing inhibition and catalepsy and high doses resulting in enhancement of activity.[8] This is likely due to preferential activation of D2/D3 autoreceptors versus postsynaptic receptors,[9] the latter of which overcomes the former to increase postsynaptic dopaminergic signaling only with high doses.
See also
References
- ↑ "(6aS)-6-Propyl-5,6,6a,7-tetrahydro-4H-dibenzo[de,gquinoline-10,11-diol"]. http://www.chemspider.com/Chemical-Structure.4470712.html.
- ↑ "Aporphines. 15. Action of aporphine alkaloids on dopaminergic mechanisms in rat brain". European Journal of Pharmacology 35 (1): 77–83. January 1976. doi:10.1016/0014-2999(76)90302-2. PMID 943290.
- ↑ "3H-N-n-propylnorapomorphine: a novel agonist ligand for central dopamine receptors". European Journal of Pharmacology 56 (4): 411–2. July 1979. doi:10.1016/0014-2999(79)90274-7. PMID 477735.
- ↑ "Comparison of the dopaminergic effects of apomorphine and (−)-N-n-propylnorapomorphine". European Journal of Pharmacology 52 (1): 1–9. November 1978. doi:10.1016/0014-2999(78)90015-8. PMID 569056.
- ↑ "Stereotypic and hypothermic effects of apomorphine and N-n-propylnorapomorphine in mice". European Journal of Pharmacology 54 (3): 273–7. March 1979. doi:10.1016/0014-2999(79)90086-4. PMID 570924.
- ↑ "Aporphines. 14 Dopaminergic and antinociceptive activity of aporphine derivatives. Synthesis of 10-hydroxyaporphines and 10-hydroxy-N-n-propylnoraporphine". Journal of Medicinal Chemistry 19 (1): 25–9. January 1976. doi:10.1021/jm00223a006. PMID 942751.
- ↑ "Penile erection induced by apomorphine and N-n-propyl-norapomorphine in rats". Archives Internationales de Pharmacodynamie et de Thérapie 242 (2): 241–7. December 1979. PMID 44457.
- ↑ "Behavioral effects of (-)10,11-methylenedioxy-N-n-propylnoraporphine, an orally effective long-acting agent active at central dopamine receptors, and analogous aporphines". Neuropharmacology 21 (10): 953–61. October 1982. doi:10.1016/0028-3908(82)90106-X. PMID 6890636.
- ↑ "N-n-propyl-norapomorphine: an extremely potent stimulant of dopamine autoreceptors". Brain Research 231 (1): 109–16. January 1982. doi:10.1016/0006-8993(82)90011-7. PMID 6799148.
 |
