Chemistry:Sumanirole
From HandWiki
Short description: Chemical compound
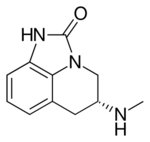 | |
| Clinical data | |
|---|---|
| Other names | PNU-95,666 |
| ATC code |
|
| Identifiers | |
| |
| CAS Number |
|
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C11H13N3O |
| Molar mass | 203.245 g·mol−1 |
| 3D model (JSmol) | |
| |
Sumanirole (PNU-95,666) is a highly selective D2 receptor full agonist, the first of its kind to be discovered.[1][2][3] It was developed for the treatment of Parkinson's disease and restless leg syndrome. While it has never been approved for medical use [4][5] it is a highly valuable tool compound for basic research to identify neurobiological mechanisms that are based on a dopamine D2-linked (vs. D1-, D3-, D4-, and D5-linked) mechanism of action.[3]
In 2004, Pfizer announced the end of their clinical development program for sumanirole, citing “recent studies that failed to sufficiently distinguish sumanirole from currently available therapies”.[6]
See also
References
- ↑ Romero AG, et al. Synthesis of the selective D2 receptor agonist PNU-95666E from D-phenylalanine using a sequential oxidative cyclization strategy. Journal of Organic Chemistry. 1997; 62(19):6582.
- ↑ "Sumanirole, a highly dopamine D2-selective receptor agonist: in vitro and in vivo pharmacological characterization and efficacy in animal models of Parkinson's disease". The Journal of Pharmacology and Experimental Therapeutics 314 (3): 1248–56. September 2005. doi:10.1124/jpet.105.084202. PMID 15980060.
- ↑ 3.0 3.1 "The effects of the dopamine D2 agonist sumanirole on prepulse inhibition in rats". European Neuropsychopharmacology 20 (6): 421–425. March 2010. doi:10.1016/j.euroneuro.2010.02.011. PMID 20346635.
- ↑ "Sumanirole versus placebo or ropinirole for the adjunctive treatment of patients with advanced Parkinson's disease". Movement Disorders 22 (4): 483–9. March 2007. doi:10.1002/mds.21191. PMID 17115380.
- ↑ "Efficacy and tolerability of sumanirole in restless legs syndrome: a phase II, randomized, double-blind, placebo-controlled, dose-response study". Sleep Medicine 8 (2): 119–27. March 2007. doi:10.1016/j.sleep.2006.05.018. PMID 17239657.
- ↑ Pfizer, Inc.. "Pfizer to Discontinue Sumanirole Development Program". Parkinson's Disease Foundation. http://www.pdf.org/en/science_news/release/pr_1216748386.
 |
