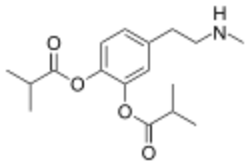
Chemistry:Ibopamine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| Chemical and physical data | |
| Formula | C17H25NO4 |
| Molar mass | 307.390 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Ibopamine is a sympathomimetic drug, designed as a prodrug of epinine (deoxyepinephrine or N-methyldopamine), used in ophthalmology.[1] It induces mydriasis.[2] It also has been investigated for use in the treatment of congestive heart failure.[3]
It acts on D1[4][5] and α-adrenergic receptors as an agonist.[6]
Ibopamine was first prepared by Casagrande and co-workers.[7]
Instilled at 2% concentration, ibopamine exhibits several functions at ocular level such as pre- and post-operative mydriatic activity, D1 dopaminergic activity, etc.[6]
Pharmacokinetics
Due to the esterases existing in the aqueous humour and ocular tissues, ibopamine can be rapidly hydrolysed to epinine which is the active molecule responsible for the mydriatic effect.[8] The epinine, an analogue of dopamine, can stimulate dopamine receptors and to a lesser degree adrenergic receptors.[9] Thus it is believed that epinine is the pharmacologically active moiety. It has been shown that the half-life of ibopamine is short to about 2 minutes in the aqueous humour owing to the fast hydrolysis.[10] So ibopamine can not be found in the aqueous humor after instillation.
Pharmacodynamics
After being hydrolysed to epinine, ibopamine is able to stimulate the alpha-adrenergic and D1 dopaminergic receptors, thereby exhibiting mydriatic effects.[11] In some randomized clinical trials, the D1 dopaminergic activity of ibopamine led to an increased production of aqueous humour and intraocular pressure (IOP) in primary open-angle glaucoma (POAG) patients.[12]
Toxicology
At systemic and local levels, ibopamine has been proved to be of low toxicity. It is well tolerated since no obvious changes to the haematological and behavioural parameters have been observed after administration.[citation needed] Ibopamine eye drop at 2% concentration, containing 1 mg of the compound, did not show any significant systemic side-effects and tachyphylaxis phenomena whereas the oral dosage is higher than 400 mg per day.[6]
Clinical Use
A fast and short-lasting mydriasis can be induced by ibopamine without systemic side-effects.
See also
- Epinine
References
- ↑ "Comparison between the 1% and 2% ibopamine provocative test in primary open-angle glaucoma patients: sensitivity, specificity and tolerability". Arquivos Brasileiros de Oftalmologia 69 (5): 695–699. 2006. doi:10.1590/S0004-27492006000500015. PMID 17187138.
- ↑ "Effects of 2% ibopamine on pupil, refraction, anterior segment anatomy and intraocular pressure". Journal of Ocular Pharmacology and Therapeutics 17 (3): 215–223. June 2001. doi:10.1089/108076801750295254. PMID 11436942.
- ↑ "Double-blind placebo-controlled study of ibopamine and digoxin in patients with mild to moderate heart failure: results of the Dutch Ibopamine Multicenter Trial (DIMT)". Journal of the American College of Cardiology 22 (6): 1564–1573. November 1993. doi:10.1016/0735-1097(93)90579-P. PMID 7901256.
- ↑ "Dopaminergic drugs in congestive heart failure: hemodynamic and neuroendocrine responses to ibopamine, dopamine, and dihydroergotoxine". Journal of Cardiovascular Pharmacology 25 (5): 732–740. May 1995. doi:10.1097/00005344-199505000-00008. PMID 7630152.
- ↑ "The effects of ibopamine on glomerular filtration rate and plasma norepinephrine remain preserved during prolonged treatment in patients with congestive heart failure". European Heart Journal 16 (7): 937–942. July 1995. doi:10.1093/oxfordjournals.eurheartj.a061028. PMID 7498209.
- ↑ 6.0 6.1 6.2 "Ibopamine stimulates α-adrenergic receptors and D1 dopaminergic receptors in the eye.". Current Drug Therapy 2 (2): 127–132. 2007. doi:10.2174/157488507780619112.
- ↑ "Synthesis and chemical properties of ibopamine and of related esters of N-substituted dopamines--synthesis of ibopamine metabolites". Arzneimittel-Forschung 36 (2A): 291–303. February 1986. PMID 3707640.
- ↑ "Ocular pharmacokinetics and pharmacodynamics in rabbits of ibopamine, a new mydriatic agent". Experimental Eye Research 56 (2): 247–254. February 1993. doi:10.1006/exer.1993.1032. PMID 8096462.
- ↑ "Effects of long-term therapy with oral ibopamine on resting hemodynamics and exercise capacity in patients with heart failure: relationship to the generation of N-methyldopamine and to plasma norepinephrine levels". Circulation 73 (4): 740–748. April 1986. doi:10.1161/01.CIR.73.4.740. PMID 3948372.
- ↑ "Topical ibopamine in the treatment of chronic ocular hypotony attributable to vitreoretinal surgery, uveitis, or penetrating trauma". American Journal of Ophthalmology 141 (3): 571–573. March 2006. doi:10.1016/j.ajo.2005.09.034. PMID 16490513.
- ↑ "Ocular pharmacokinetics and pharmacodynamics in rabbits of ibopamine, a new mydriatic agent". Experimental Eye Research 56 (2): 247–254. February 1993. doi:10.1006/exer.1993.1032. PMID 8096462.
- ↑ "Ibopamine in glaucoma diagnostics: a new pharmacological provocative test". International Ophthalmology 20 (1–3): 151–155. 1996-01-01. doi:10.1007/BF00212962. PMID 9112180.
 |
