Chemistry:SN 35210
 | |
| Clinical data | |
|---|---|
| ATC code |
|
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
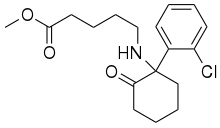
| Formula | C18H24ClNO3 |
| Molar mass | 337.84 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
SN 35210 is an arylcyclohexylamine dissociative anesthetic drug. It was derived from ketamine with the intention of producing a shorter acting agent more suitable to be used as a stand-alone drug, whereas ketamine itself generally has to be used in combination with other drugs such as midazolam to minimise the occurrence of emergence reactions due to its hallucinogenic side effects. In common with other short-acting anaesthetic drugs such as remifentanil and remimazolam, SN 35210 has had the chemical structure modified to incorporate a methyl ester group which is rapidly metabolised to a carboxylic acid, producing an inactive compound and thus rapidly terminating the effects of the drug. It was selected for development from a series of structurally related alkyl esters due to having the shortest duration of action and the most similar pharmacological profile to ketamine itself.[1][2][3][4][5]
References
- ↑ "Structure-activity relationships for ketamine esters as short-acting anaesthetics". Bioorganic & Medicinal Chemistry 21 (17): 5098–106. September 2013. doi:10.1016/j.bmc.2013.06.047. PMID 23876339.
- ↑ "Development of Rapidly Metabolized and Ultra-Short-Acting Ketamine Analogs". Anesthesia and Analgesia 121 (4): 925–33. October 2015. doi:10.1213/ANE.0000000000000719. PMID 25822925.
- ↑ "Determination of the Hypnotic Potency in Rats of the Novel Ketamine Ester Analogue SN 35210". Pharmacology 96 (5–6): 226–32. 2015. doi:10.1159/000439598. PMID 26352278.
- ↑ "Transcriptional changes in response to ketamine ester-analogs SN 35210 and SN 35563 in the rat brain". BMC Genomics 20 (1): 281. April 2019. doi:10.1186/s12864-019-5649-6. PMID 30971208.
- ↑ "KEA-1010, a ketamine ester analogue, retains analgesic and sedative potency but is devoid of Psychomimetic effects". BMC Pharmacology & Toxicology 20 (1): 85. December 2019. doi:10.1186/s40360-019-0374-y. PMID 31856925.
 |
