Chemistry:Tetrafluoroberyllate
| |
| Names | |
|---|---|
| IUPAC name | |
| Systematic IUPAC name
Tetrafluoroberyllate(2−)[5] | |
| Other names
Tetrafluoroberyllate[1]
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChemSpider | |
| 2035[10] | |
PubChem CID
|
|
| UNII | |
| |
| Properties | |
| [BeF 4]2− | |
| Molar mass | 85.0057958 g·mol−1 |
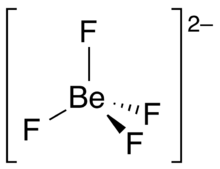
| Structure | |
| Td | |
| tetrahedral | |
| Related compounds | |
Related isoelectronic
|
|
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
Tetrafluoroberyllate or orthofluoroberyllate is an anion with the chemical formula [[[Beryllium|Be]]F
4]2−. It contains beryllium and fluorine. This fluoroanion has a tetrahedral shape, with the four fluorine atoms surrounding a central beryllium atom. It has the same size, charge, and outer electron structure as sulfate SO2−
4. Therefore, many compounds that contain sulfate have equivalents with tetrafluoroberyllate. Examples of these are the langbeinites, and Tutton's salts.
Properties
The Be–F bond length is between 145 and 153 pm. The beryllium is sp3 hybridized, leading to a longer bond than in BeF
2, where beryllium is sp hybridized.[11] In trifluoroberyllates, there are actually BeF
4 tetrahedra arranged in a triangle, so that three fluorine atoms are shared on two tetrahedra each, resulting in a formula of Be
3F
9.[12]
In the tetrafluoroberyllates, the tetrahedra can rotate to various degrees. At room temperature, they are hindered from moving. But as temperature increases, they can rotate around the threefold axis, (i.e. a line through one fluorine atom and the beryllium atom) with a potential barrier of 12.5 kcal/mol (52 kJ/mol). At higher temperatures, the movement can become isotropic (not limited to rotation on one axis) with a potential barrier of 14.5 kcal/mol (61 kJ/mol).[11]
Similar compounds have magnesium or zinc in a similar position as beryllium, e.g. K
2[MgF
4] (potassium tetrafluoromagnesate) or [NH
4]
2[ZnF
4] (ammonium tetrafluorozincate) but these are not as stable.[12]
Tetrafluoroberyllate has a biological effect by inhibiting F-ATPase adenosine triphosphate producing enzymes in mitochondria and bacteria. It does this by attempting to react with adenosine diphosphate because it resembles phosphate. However once it does this it remains stuck in the F1 part of the enzyme and inhibits it from further function.[13]
Simple salts
| Name | Chemical formula | Molar mass (g/mol) | CAS number | Crystal system | Density (g/cm3) | Melting point (°C) | Solubility in water (g/(100 ml)) |
|---|---|---|---|---|---|---|---|
| lithium tetrafluoroberyllate | Li 2[BeF 4] |
98.89 | 2.167[14] | 472 °C[15] | slight (1.25 at 20 °C, 5.78 at 40 °C)[16] | ||
| sodium tetrafluoroberyllate | Na 2BeF 4 |
130.985333 | 13871-27-7 | Orthorhombic[17] | 2.47 | 575 °C | slight (1.33 at 0 °C, 1.44 at 20 °C, 2.73 at 90 °C)[18] |
| potassium tetrafluoroberyllate | K 2BeF 4 |
163.20 | 7787-50-0 | orthorhombic a = 5.691 Å, b = 7.278 Å, c = 9.896 Å[19] as for strontium orthosilicate[12] | 2.64[19] | ||
| potassium tetrafluoroberyllate dihydrate | K 2BeF 4 · 2H2O |
199.233 | |||||
| ammonium tetrafluoroberyllate | (NH 4) 2BeF 4 |
121.0827 | 14874-86-3 | orthorhombic a = 5.91 Å, b = 7.64 Å, c = 10.43 Å | 1.71 | decomposes 280 °C[20] | 32.3 at 25 °C[21] |
| rubidium tetrafluoroberyllate | Rb 2BeF 4 |
255.941 | orthorhombic a = 5.87 Å, b = 7.649 Å, c = 10.184 Å[19] | 3.72[19] | |||
| caesium tetrafluoroberyllate | Cs 2BeF 4 |
350.8167 | orthorhomic a = 8.03 Å, b = 10.81 Å, c = 0.622 Å | 4.32 | |||
| thallium tetrafluoroberyllate | Tl 2BeF 4 |
493.7724 | orthorhombic a = 7.7238 Å, b = 5.9022 Å, c = 10.4499 Å[22] | 6.884[22] | |||
| silver tetrafluoroberyllate | Ag 2BeF 4 |
300.7422 | |||||
| magnesium tetrafluoroberyllate | MgBeF 4 |
109.3108 | |||||
| calcium tetrafluoroberyllate | CaBeF 4 |
125.08 | 2.959[23] | ||||
| strontium tetrafluoroberyllate | SrBeF 4 |
172.6 | orthorhombic a = 5.291 Å, b = 6.787 Å, c = 8.307 Å | 3.84 | insoluble | ||
| barium tetrafluoroberyllate | BaBeF 4 |
222.333 | 4.17[14] | insoluble | |||
| radium tetrafluoroberyllate | RaBeF 4[24] |
311.005795 | insoluble | ||||
| hexaqua ferrous tetrafluoroberyllate | FeBeF 4 · 6H2O[25] |
||||||
| heptaqua ferrous tetrafluoroberyllate | FeBeF 4 · 7H2O[23] |
1.894 | |||||
| heptaqua nickel tetrafluoroberyllate | NiBeF 4 · 7H2O[23] |
||||||
| hexaqua nickel tetrafluoroberyllate | NiBeF 4 · 6H2O[23] |
||||||
| heptaqua cobalt tetrafluoroberyllate | CoBeF 4 · 7H2O[23] |
1.867 | |||||
| hexaqua cobalt tetrafluoroberyllate | CoBeF 4 · 6H2O[23] |
1.891 | |||||
| pentaqua copper tetrafluoroberyllate | CuBeF 4 · 5H2O[23] |
||||||
| heptaqua zinc tetrafluoroberyllate | ZnBeFe 4 · 7H2O[23] |
||||||
| lead tetrafluoroberyllate | PbBeF 4 |
292.2 | 6.135[14] | ||||
| hydrazinium tetrafluoroberyllate | N 2H 6BeF 4 |
119.0668 | a = 5.58 Å, b = 7.337 Å, c = 9.928 Å, α = 90°, β = 98.22°, γ = 90°[19] | ||||
| triglycine tetrafluoroberyllate | (NH 2CH 2COOH) 3 · H 2BeF 4 |
312.221 | 2396-72-7 | monoclinic[26][27] | |||
| ethylene diamine fluoroberyllate | (NH 2CH 2CH 2NH 2) · H 2BeF 4[28] |
decomposes 330 °C | |||||
| propylenediamine tetrafluoroberyllate | (NH 2CH 2CH 2CH 2NH 2) · H 2BeF 4[29] |
||||||
| propylene-1,2-diamine tetrafluoroberyllate | (NH 2CH(CH 3)CH 2NH 2) · H 2BeF 4[28] |
monoclinic a = 5.535 Å, b = 13.560 Å, c = 9.6048 Å, β = 106.73 Å, V = 690.4 Å3, Z = 4[30] | 1.55 | ||||
| benzidine fluoroberyllate | (NH 2C 6H 4C 6H 4NH 2) · H 2BeF 4[28] |
ins | |||||
| tetramethyl ammonium tetrafluoroberyllate | [N(CH 3) 4] 2BeF 4[14] |
||||||
| tetramine silver tetrafluoroberyllate | [Ag(NH 3) 2] 2BeF 4[31] |
||||||
| [Cu(NH 3) 2] 2BeF 4[31] |
|||||||
| [Cu(NH 3) 4] 2BeF 4 · H2O[31] |
|||||||
| [Zn(NH 3) 4] 2BeF 4[31] |
|||||||
| [Cd(NH 3) 4] 2BeF 4[31] |
|||||||
| [Ni(NH 3) 6] 2BeF 4[31] |
|||||||
| [Ni(NH 3) 4] 2BeF 4 · 2H2O[31] |
|||||||
| [Ni(NH 3) 2] 2BeF 4[31] |
|||||||
| [Co(NH 3) 6] 2BeF 4 · 3H2O[31] |
Sodium tetrafluoroberyllate has several crystalline forms. Below 220 °C it takes the same form as orthorhombic olivine, and this is called γ phase. Between 220 °C and 320 °C it is in the α′ form. When temperature is raised above 320 °C it changes to the hexagonal α form. When cooled the α′ form changes to β form at 110 °C and this can be cooled to 70 °C before changing back to the γ form.[32] It can be formed by melting sodium fluoride and beryllium fluoride.[32] The gas above molten sodium tetrafluoroberyllate contains BeF
2 and NaF gas.[11]
Lithium tetrafluoroberyllate takes on the same crystal form as the mineral phenacite. As a liquid it is proposed for the molten salt reactor, in which it is called FLiBe. The liquid salt has a high specific heat, similar to that of water. The molten salt has a very similar density to the solid. The solid has continuous void channels through it, which reduces its density.[15] Li
2BeF
4 can be crystallised from aqueous solution using (NH
4)
2BeF
4 and LiCl.[33]
Potassium tetrafluoroberyllate has the same structure as anhydrous potassium sulfate, as does rubidium and caesium tetrafluoroberyllate. Potassium tetrafluoroberyllate can make solid solutions with potassium sulfate.[11] It can be used as a starting point to make the non-linear optic crystal KBe
2BO
3F
2 which has the highest power handling capacity and shortest UV performance of any borate.[34] It is quite soluble in water, so beryllium can be extracted from soil in this form.[35]
Ammonium tetrafluoroberyllate decomposes on heating by losing NH
4F vapour, progressively forming NH
4BeF
3, then NH
4Be
2F
5 and finally BeF
2.[11]
Thallium tetrafluoroberyllate can be made by dissolving beryllium fluoride and thallium carbonate together in hydrofluoric acid and then evaporating the solution.[22]
Radium tetrafluoroberyllate is used as a standard neutron source. The alpha particles from the radium cause neutrons to be emitted from the beryllium. It is precipitated from a radium chloride solution mixed with potassium tetrafluoroberyllate.[12]
Magnesium tetrafluoroberyllate can be precipitated from a hot saturated solution of ammonium tetrafluoroberyllate and a magnesium salt.[11] However, if the temperature reaches boiling point MgF
2 is precipitated instead.[36]
Calcium tetrafluoroberyllate resembles zircon in the way it melts and crystallises.[11]
Strontium tetrafluoroberyllate can be made in several forms. The γ form is produced by cooling a melt of SrF
2 and Be
2 and the β form is made by precipitating from a water solution. When melted and heated to 850–1145 °C, Be
2 gas evaporates leaving behind molten SrF
2.[11]
The barium tetrafluoroberyllate is very insoluble and can be used for gravimetric analysis of beryllium.[11]
H
2BeF
4 is an acid that can be produced from Ag
2BeF
4 and HCl. It only exists in aqueous solution.[11]
Triglycine tetrafluoroberyllate (TGFB) is ferroelectric with a transition point of 70 °C.[26] The crystals can be formed by dissolving BeF
2 in water, adding HF and then glycine. When the solution is cooled triglycine tetrafluoroberyllate forms. Cs
2BeF
4 and Tl
2BeF
4 in the solution reduce growth on the 001 direction so that tabular shaped crystals of TGFB form. The thallium compound can cut growth on the 001 axis by 99%.[37]
Double salts
Tuttons salts
The Tuttons salt (NH4)2Mn(BeF4)2·6(H2O) is made from a solution of NH4BeF3 mixed with NH4MnF3.[11] The equivalent of alums are hard to make because the trivalent ion will often form a complex with fluoride in preference to the beryllium fluoride. However the violet coloured acid and rubidium chrome alum exist at chilly temperatures for a few hours.[38]
Tutton's salts (also called schoenites) containing magnesium with fluoroberyllate are difficult to produce, as the solutions tend to precipitate insoluble MgF2.[39]
| name | formula | molecular weight | CAS | crystal form | density | melting point | solubility g/100ml |
|---|---|---|---|---|---|---|---|
| potassium lithium tetrafluoroberyllate | KLiBeF4 | 131.05 | P63, a = 8.781 Å, b = 5.070 Å c = 8.566 Å[40] | ||||
| rubidium lithium tetrafluoroberyllate | RbLiBeF4 | 177.41 | P6322, a = 8.980 Å, b = 5.185 Å c = 8.751 Å[40] | ||||
| caesium lithium tetrafluoroberyllate | CsLiBeF4 | 224.852 | P21/n, a = 9.328 Å b = 5.356 Å, c = 8.736 Å, γ = 89.82°[40] | ||||
| acid chromium fluoroberyllate tetracosihydrate | H2Cr2(BeF4)4·24H2O[38] | 878.40 | |||||
| ammonium chromium fluoroberyllate tetracosihydrate | (NH4)2Cr2(BeF4)4·24H2O[38] | 912.46 | |||||
| rubidium chromium fluoroberyllate tetracosihydrate | Rb2Cr2(BeF4)4·24H2O[38] | 1047.32 | |||||
| manganese ammonium fluoroberyllate hydrate | (NH4)2Mn(BeF4)2·6H2O[39] | 369.118 | 1.758[41] | ||||
| Rb2Fe(BeF4)2·6H2O[39] | 504.884 | ||||||
| ferrous ammonium fluoroberyllate hydrate | (NH4)2Fe(BeF4)2·6H2O[39] | 370.025[41] | |||||
| nickel potassium fluoroberyllate hydrate | K2Ni(BeF4)2·6H2O[39] | 414.913[41] | |||||
| nickel rubidium fluoroberyllate hydrate | Rb2Ni(BeF4)2·6H2O[39] | 507.732 | |||||
| Cs2Ni(BeF4)2·6H2O[39] | 602.608 | ||||||
| nickel ammonium fluoroberyllate hydrate | (NH4)2Ni(BeF4)2·6H2O[39] | 372.874 | P21/a, a = 9.201 Å, b = 12.482 Å, c = 6.142 Å, β = 106.57 Å, V = 676.0 Å3 Z = 2[42] | 1.843[41] | |||
| cobalt potassium fluoroberyllate hydrate | K2Co(BeF4)2·6H2O[39] | 415.233[41] | |||||
| cobalt rubidium fluoroberyllate hydrate | Rb2Co(BeF4)2·6H2O[39] | 507.972 | |||||
| cobalt ammonium fluoroberyllate hydrate | (NH4)2Co(BeF4)2·6H2O[39] | 372.874 | 1.821[41] | ||||
| copper rubidium fluoroberyllate hydrate | Rb2Cu(BeF4)2·6H2O[39] | 512.585 | |||||
| copper ammonium fluoroberyllate hydrate | (NH4)2Cu(BeF4)2·6H2O[39] | 377.726 | 1.858[41] | ||||
| zinc rubidium fluoroberyllate hydrate | Rb2Zn(BeF4)2·6H2O[39] | 514.42 | |||||
| zinc ammonium fluoroberyllate hydrate | (NH4)2Zn(BeF4)2·6H2O[39] | 379.56 | 1.859[41] | ||||
| cadmium rubidium fluoroberyllate hydrate | Rb2Cd(BeF4)2·6H2O[39] | 561.45 | |||||
| cadmium ammonium fluoroberyllate hydrate | (NH4)2Cd(BeF4)2·6H2O[39] | 426.591 |
Alums
Tetrafluoroberyllate salts equivalent to alums also exist with formula MABF4·12H2O, where M is univalent, and A trivalent. These are not common as fluoride often form insoluble products with the trivalent ions. Methods to produce these include evaporating mixed fluoride solutions under reduced pressure at 0 °C, or dissolving beryllium and other metal hydroxides in hydrofluoric acid at room temperature, cooled, and them mixing with cold ethyl alcohol, causing cooling and crystallisation.[43] The unit cell dimensions are slightly smaller (by 0.03–0.05 Å) than the corresponding sulfate alums.[43]
| name | formula | molecular weight | CAS | crystal form | density | melting point | solubility g/100ml |
|---|---|---|---|---|---|---|---|
| ammonium aluminium tetrafluoroberyllate alum | NH4AlBeF4·12H2O | [43] | |||||
| potassium aluminium tetrafluoroberyllate alum | KAlBeF4·12H2O | [43] | |||||
| potassium chromium tetrafluoroberyllate alum | KCrBeF4·12H2O | [43] | |||||
| ammonium chromium tetrafluoroberyllate alum | NH4CrBeF4·12H2O | cubic a = 12.218 Å, Z = 4[43] | |||||
| rubidium chromium tetrafluoroberyllate alum | RbCrBeF4·12H2O | 12.214 Å[43] | |||||
| caesium chromium tetrafluoroberyllate alum | CsCrBeF4·12H2O | 12.323 Å[43] | |||||
| thallium chromium tetrafluoroberyllate alum | TlCrBeF4·12H2O | 12.195 Å[43] | |||||
| rubidium iron tetrafluoroberyllate alum | RbFeBeF4·12H2O | [43] | |||||
| caesium iron tetrafluoroberyllate alum | CsFeBeF4·12H2O | [43] | |||||
| monomethyl chromium tetrafluoroberyllate alum | CH3NH3CrBeF4·12H2O | 12.496 Å[44] | |||||
| guanidium chromium tetrafluoroberyllate alum | C(NH2)3CrBeF4·12H2O | 12.538 Å[44] | on heating forms a rhombohedral hexahydrate stable from 30 °C to 90 °C |
References
- ↑ "Beryllium tetrafluoride". https://pubchem.ncbi.nlm.nih.gov/compound/5834288#section=Depositor-Supplied-Synonyms. Retrieved 30 January 2019. "Depositor-Supplied Synonyms beryllium tetrafluoride Tetrafluoroberyllate"
- ↑ "tetrafluoroberyllate(2−) (CHEBI:30497)" (table). 26 January 2009. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:30497. Retrieved 30 January 2019. "Synonyms Sources tetrafluoroberyllate(2−) IUPAC"
- ↑ "Tetrafluoroberyllate(2−)". p. Names. http://www.chemspider.com/Chemical-Structure.15238330.html. Retrieved 30 January 2019. "Tetrafluoroberyllate(2-) [ACD/IUPAC Name]"
- ↑ "tetrafluoroberyllate(2−) (CHEBI:30497)". 26 January 2009. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:30497. Retrieved 30 January 2019. "IUPAC Name tetrafluoridoberyllate(2−)"
- ↑ "Tetrafluoroberyllate(2−)". p. More details. http://www.chemspider.com/Chemical-Structure.15238330.html. Retrieved 30 January 2019. "Systematic name Tetrafluoroberyllate(2−)"
- ↑ "Tetrafluoroberyllate(2−)". p. Data Sources. http://www.chemspider.com/Chemical-Structure.15238330.html. Retrieved 30 January 2019. "30497"
- ↑ "tetrafluoroberyllate(2-) (CHEBI:30497)". 26 January 2009. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:30497. Retrieved 30 January 2019. "ChEBI ID CHEBI:30497"
- ↑ "Tetrafluoroberyllate(2−)". p. More details. http://www.chemspider.com/Chemical-Structure.15238330.html. Retrieved 30 January 2019. "SMILES [Be-2](F)(F)(F)F"
- ↑ "tetrafluoroberyllate(2−) (CHEBI:30497)" (table). 26 January 2009. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:30497. Retrieved 30 January 2019. "SMILES F[Be--](F)(F)F"
- ↑ "tetrafluoroberyllate(2−) (CHEBI:30497)" (table). 26 January 2009. https://www.ebi.ac.uk/chebi/searchId.do?chebiId=CHEBI:30497. Retrieved 30 January 2019. "Registry Number Type Source 2035 Gmelin Registry Number Gmelin"
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 Emeléus, Harry Julius; Sharpe, A. G. (1972-12-06). ADVANCES IN INORGANIC CHEMISTRY AND RADIOCHEMISTRY. Academic Press. pp. 271–275. ISBN 9780080578637. https://books.google.com/books?id=VupzlLU9NB0C&pg=PA271. Retrieved 13 July 2013.
- ↑ 12.0 12.1 12.2 12.3 Simons, J.H. (1954-01-01). Fluorine Chemistry. Elsevier. p. 5. ISBN 9780323145435. https://books.google.com/books?id=ott67ONZPKwC&pg=PA5. Retrieved 13 July 2013.
- ↑ Lunardi, Joel; Dupuis, Alain; Garin, Jerome; Issartel, Jean-Paul; Laurent, Michel; Peinnequin, Andre; Vignais, Pierre (1992). Fluoroaluminum and Fluoroberyllium Complexes as Probes of the Catalytic Sites of Mitochondrial F1-ATPases. UNESCO. pp. 59–69. ISBN 9783034873154. https://books.google.com/books?id=1CH3BwAAQBAJ&pg=PA59.
- ↑ 14.0 14.1 14.2 14.3 Rây, Nirmalendu Nath (1931). "Fluoberyllate und ihre Analogie mit Sulfaten. I". Zeitschrift für anorganische und allgemeine Chemie 201 (1): 289–300. doi:10.1002/zaac.19312010126. ISSN 0863-1786.
- ↑ 15.0 15.1 Douglas, Thomas B.; William H. Payne (May 20, 1969). "Measured Solid Enthalpy and Derived Thermodynamic Properties of and Liquid Lithium Tetrafluoroberyllate, Li2BeF4 from 273 to 900 K". Journal of Research of the National Bureau of Standards Section A (Washington, D.C.: Institute for Basic Standards, National Bureau of Standards) 73A (5).
- ↑ Perfect, F. H. (1952). "FURTHER OBSERVATIONS ON THE SIMILARITIES OF FLUOBERYLLATE AND SULPHATE IONS". Proceedings of the Pennsylvania Academy of Science 26: 54-65.
- ↑ Furuhashi, Koushi; Junko Habasaki; Isao Okada (1986). "A molecular dynamics study of the structures and dynamic properties of molten NaBeF3and Na2BeF4". Molecular Physics 59 (6): 1329–1344. doi:10.1080/00268978600102761. ISSN 0026-8976. Bibcode: 1986MolPh..59.1329F.
- ↑ Perry, Dale L. (2011-05-19). Handbook of Inorganic Compounds, Second Edition. Taylor & Francis. p. 394. ISBN 9781439814611. https://books.google.com/books?id=SFD30BvPBhoC&pg=PA50. Retrieved 13 July 2013.
- ↑ 19.0 19.1 19.2 19.3 19.4 Villars, P.. "AtomWork Materials Database". Nationional Institute of Materials Science. http://crystdb.nims.go.jp/crystdb/search-materials. Retrieved 17 July 2013.
- ↑ Andreev, A. A.; A. N. D’yachenko, R. I. Kraidenko (2011). "Fluorination of beryllium concentrates with ammonium fluorides". Russian Journal of Applied Chemistry 81 (2): 178–182. doi:10.1134/S1070427208020043. ISSN 1070-4272.
- ↑ Dyachenko, A.N.; Kraydenko, R.I.; Petlin, I.V.; Malyutin, L.N. (2016). "The Research of (NH4)2BeF4 Solution Purification Effectiveness". Procedia Engineering 152: 51–58. doi:10.1016/j.proeng.2016.07.624.

- ↑ 22.0 22.1 22.2 da Silva, Iván; González Silgo, Cristina; González Platas, Javier; Rodríguez Carvajal, Juan; Martínez Sarrión, María Luisa; Mestres, Lourdes (2005). "Powder neutron diffraction of Tl2BeF4 at six temperatures from room temperature to 1.5 K". Acta Crystallographica Section C 61 (12): i113–i116. doi:10.1107/S010827010503249X. ISSN 0108-2701. PMID 16330826.
- ↑ 23.0 23.1 23.2 23.3 23.4 23.5 23.6 23.7 Rây, Nirmalendu Nath (1932). "Fluoberyllate und ihre Analogie mit den Sulfaten. II. Fluoberyllate einiger zweiwertiger Metalle" (in German). Zeitschrift für anorganische und allgemeine Chemie 205 (3): 257–267. doi:10.1002/zaac.19322050307. ISSN 0863-1786.
- ↑ Sastri, Malladi Narasimha (1958). Radiochemical measurements on neutron sources (PDF) (Thesis). Durham University. pp. 18–20.
- ↑ Kaduk, J. A. (2019). "Section 4.9.4. Chemical reasonableness". in Gilmore, C. J.; Kaduk, J. A.; Schenk, H.. International Tables for Crystallography Volume H Powder diffraction. pp. 496–508. https://onlinelibrary.wiley.com/iucr/itc/Ha/ch4o9v0001/sec4o9o4/.
- ↑ 26.0 26.1 Zarembovskaya, T. A.; V. M. Varikash; P. A. Pupkevich (1972). "Thermal expansion of triglycine fluoroberyllate crystals near the ferroelectric transition point". Soviet Physics Journal 15 (6): 920–922. doi:10.1007/BF00912245. ISSN 0038-5697. Bibcode: 1972SvPhJ..15..920Z.
- ↑ Ghazaryan, V.V.; Fleck, M.; Petrosyan, A.M. (September 2014). "New chemical analogs of triglycine sulfate". Journal of Crystal Growth 401: 857–862. doi:10.1016/j.jcrysgro.2013.11.054. Bibcode: 2014JCrGr.401..857G.
- ↑ 28.0 28.1 28.2 Ghosh, Amiya Kanti (1959). "Fluoberyllates of Organic Bases. I". Zeitschrift für anorganische und allgemeine Chemie 300 (1–2): 98–101. doi:10.1002/zaac.19593000110. ISSN 0044-2313.
- ↑ Kanti Ghosh, Amiya; Nirmalendu Nath Ráy (1959). "Fluoberyllates and their Analogy with Sulphates. XII. Complex Compounds of Zinc and Cadmium Fluoberyllate with Organic Bases". Zeitschrift für anorganische und allgemeine Chemie 300 (1–2): 109–112. doi:10.1002/zaac.19593000112. ISSN 0044-2313.
- ↑ Gerrard, Lee A.; Weller, Mark T. (30 September 2002). "Propane-1,2-diammonium tetrafluoroberyllate". Acta Crystallographica Section C 58 (10): m504–m505. doi:10.1107/S0108270102015718. PMID 12359927.
- ↑ 31.0 31.1 31.2 31.3 31.4 31.5 31.6 31.7 31.8 Rây, Nirmalendunath (1939). "Fluoberyllate und ihre Analogie mit Sulfaten. VI. Die Fluoberyllate von Metallamminkomplexen". Zeitschrift für anorganische und allgemeine Chemie 241 (2–3): 165–171. doi:10.1002/zaac.19392410203. ISSN 0863-1786.
- ↑ 32.0 32.1 Holm, J. L.; K. Lønvik (1982). "Studies of the polymorphic transformations of dicalcium silicate (Ca2SiO4) and sodium tetrafluoroberyllate (Na2BeF4) by Thermosonimetry and differential scanning calorimetry". Journal of Thermal Analysis 25 (1): 109–115. doi:10.1007/BF01913059. ISSN 0368-4466.
- ↑ Simons, J.H. (1964-01-01). Fluorine Chemistry. Elsevier. pp. 20–22. ISBN 9780323147248. https://books.google.com/books?id=9AbqU4cI93wC&pg=PA21. Retrieved 13 July 2013.
- ↑ Karas, George V. (2005). New Developments in Crystal Growth Research. Nova Publishers. pp. 24–26. ISBN 9781594545399. https://books.google.com/books?id=PjpWUwI7VSgC&pg=PA26. Retrieved 14 July 2013.
- ↑ Stone, John (May 2004). "Extraction ofBeryllium-10 from Soil by Fusion". http://depts.washington.edu/cosmolab/chem/fusionmethod.pdf. Retrieved 14 July 2013.
- ↑ Walsh, Kenneth A. (2009-01-01). Beryllium Chemistry and Processing. ASM International. p. 100. ISBN 9780871707215. https://books.google.com/books?id=3-GbhmSfyeYC&pg=PA100. Retrieved 14 July 2013.
- ↑ Wieder, H.H.; C.R. Parkerson (1966). "Some ferroelectric and dielectric properties of triglycine fluoberyllate". Journal of Physics and Chemistry of Solids 27 (2): 247–252. doi:10.1016/0022-3697(66)90029-1. ISSN 0022-3697. Bibcode: 1966JPCS...27..247W.
- ↑ 38.0 38.1 38.2 38.3 Ghosh, Amiya Kanti (1959). "Complex Chromic Fluoberyllates. I". Zeitschrift für anorganische und allgemeine Chemie 300 (1–2): 102–108. doi:10.1002/zaac.19593000111. ISSN 0044-2313.
- ↑ 39.00 39.01 39.02 39.03 39.04 39.05 39.06 39.07 39.08 39.09 39.10 39.11 39.12 39.13 39.14 39.15 39.16 Rây, Nirmalendunath (1936). "Fluoberyllate und ihre Analogie mit Sulfaten. IV. Doppelsalze mit Rubidium- und Cäsiumfluoberyllaten" (in German). Zeitschrift für anorganische und allgemeine Chemie 227 (1): 32–36. doi:10.1002/zaac.19362270105. ISSN 0863-1786.
- ↑ 40.0 40.1 40.2 Hahn, Th.; G. Lohre; S. J. Chung (1969). "A new tetrahedral framework structure in sulfates and fluoberyllates". Die Naturwissenschaften 56 (9): 459. doi:10.1007/bf00601063. ISSN 0028-1042. Bibcode: 1969NW.....56Q.459H.
- ↑ 41.0 41.1 41.2 41.3 41.4 41.5 41.6 41.7 Nath Rây, Nirmalendu (1932). "Fluoberyllate und ihre Analogie mit Sulfaten. III. Doppelsalze der Fluoberyllate" (in German). Zeitschrift für anorganische und allgemeine Chemie 206 (2): 209–216. doi:10.1002/zaac.19322060209. ISSN 0863-1786.
- ↑ Montgomery, H. (15 September 1980). "Diammonium nickel bis(tetrafluoroberyllate)hexahydrate". Acta Crystallographica Section B 36 (9): 2121–2123. doi:10.1107/S0567740880008060. http://journals.iucr.org/b/issues/1980/09/00/a19083/a19083.pdf.
- ↑ 43.00 43.01 43.02 43.03 43.04 43.05 43.06 43.07 43.08 43.09 43.10 Lari-Lavassani, Abbasse; Avinens, Christian; Cot, Louis (19 May 1969). "Préparation et étude radiocristallographique des aluns fluorobéryllates de chrome" (in fr). Comptes rendus hebdomadaires des séances de l'Académie des sciences (Paris) C268: 1782–1784. https://gallica.bnf.fr/ark:/12148/bpt6k4802761/f1805.image.
- ↑ 44.0 44.1 Lari-Lavassani, Abbasse; Avinens, Christian; Cot, Louis (15 June 1970). "Sur l'existence et la cristallographie de quelques nouveaux fluorobéryllates doubles de chrome [CH3NH3Cr(BeF4)2·12H2O, [C(NH2)3]Cr(BeF4)2·12H2O et [C(NH2)3]Cr(BeF4)2·6H2O"] (in fr). Comptes rendus hebdomadaires des séances de l'Académie des sciences (Paris) C270: 1973–1975. https://gallica.bnf.fr/ark:/12148/bpt6k480278s/f2016.image.
 |

