Chemistry:Cyclophosphamide
 | |
 | |
| Clinical data | |
|---|---|
| Pronunciation | /ˌsaɪkloʊˈfɒsfəˌmaɪd, -lə-/[1][2] |
| Trade names | Lyophilized Cytoxan, Endoxan, Cytoxan, Neosar, Procytox, Revimmune, Cycloblastin |
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a682080 |
| Pregnancy category |
|
| Routes of administration | By mouth, by injection into a vein |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Bioavailability | >75% (by mouth) |
| Protein binding | >60% |
| Metabolism | Liver |
| Elimination half-life | 3–12 hours |
| Excretion | Kidney |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
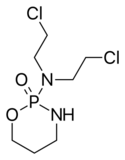
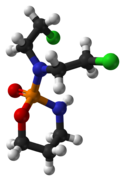
| Formula | C7H15Cl2N2O2P |
| Molar mass | 261.08 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 2 °C (36 °F) |
| |
| |
| (verify) | |
Cyclophosphamide (CP), also known as cytophosphane among other names,[3] is a medication used as chemotherapy and to suppress the immune system.[4] As chemotherapy it is used to treat lymphoma, multiple myeloma, leukemia, ovarian cancer, breast cancer, small cell lung cancer, neuroblastoma, and sarcoma.[4] As an immune suppressor it is used in nephrotic syndrome, granulomatosis with polyangiitis, and following organ transplant, among other conditions.[4][5] It is taken by mouth or injection into a vein.[4]
Most people develop side effects.[4] Common side effects include low white blood cell counts, loss of appetite, vomiting, hair loss, and bleeding from the bladder.[4] Other severe side effects include an increased future risk of cancer, infertility, allergic reactions, and pulmonary fibrosis.[4] Cyclophosphamide is in the alkylating agent and nitrogen mustard family of medications.[4] It is believed to work by interfering with the duplication of DNA and the creation of RNA.[4]
Cyclophosphamide was approved for medical use in the United States in 1959.[4] It is on the World Health Organization's List of Essential Medicines.[6]
Medical uses
Cyclophosphamide is used to treat cancers and autoimmune diseases. It is used to quickly control the disease. Due to its toxicity, it is replaced as soon as possible by less toxic drugs. Regular and frequent laboratory evaluations are required to monitor kidney function, avoid drug-induced bladder complications and screen for bone marrow toxicity.
Cancer
The main use of cyclophosphamide is with other chemotherapy agents in the treatment of lymphomas, some forms of brain cancer, neuroblastoma, leukemia and some solid tumors.[7]
Autoimmune diseases
Cyclophosphamide decreases the immune system's response, and although concerns about toxicity restrict its use to patients with severe disease, it remains an important treatment for life-threatening autoimmune diseases where disease-modifying antirheumatic drugs (DMARDs) have been ineffective. For example, systemic lupus erythematosus with severe lupus nephritis may respond to pulsed cyclophosphamide. Cyclophosphamide is also used to treat minimal change disease,[8] severe rheumatoid arthritis, granulomatosis with polyangiitis,[5] Goodpasture syndrome[9] and multiple sclerosis.[10]
Because of its potential side effects such as amenorrhea or ovarian failure, cyclophosphamide is used for early phases of treatment and later substituted by other medications, such as mycophenolic acid or ACA.[11][12]
AL amyloidosis
Cyclophosphamide, used in combination with thalidomide or lenalidomide and dexamethasone has documented efficacy as an off-label treatment of AL amyloidosis. It appears to be an alternative to the more traditional treatment with melphalan in people who are ill-suited for autologous stem cell transplant.[13][7]
Graft-versus-host disease
Graft-versus-host disease (GVHD) is a major barrier for allogeneic stem cell transplant because of the immune reactions of donor T cell against the person receiving them. GVHD can often be avoided by T-cell depletion of the graft.[14] The use of a high dose cyclophosphamide post-transplant in a half matched or haploidentical donor hematopoietic stem cell transplantation reduces GVHD, even after using a reduced conditioning regimen.[15][16]
Contraindications
Like other alkylating agents, cyclophosphamide is teratogenic and contraindicated in pregnant women (pregnancy category D) except for life-threatening circumstances in the mother. Additional relative contraindications to the use of cyclophosphamide include lactation, active infection, neutropenia or bladder toxicity.[7]
Cyclophosphamide is a pregnancy category D drug and causes birth defects. First trimester exposure to cyclophosphamide for the treatment of cancer or lupus displays a pattern of anomalies labeled "cyclophosphamide embryopathy", including growth restriction, ear and facial abnormalities, absence of digits and hypoplastic limbs.[17]
Side effects
Adverse drug reactions from cyclophosphamide are related to the cumulative medication dose and include chemotherapy-induced nausea and vomiting,[18] bone marrow suppression,[19] stomach ache, hemorrhagic cystitis, diarrhea, darkening of the skin/nails, alopecia (hair loss) or thinning of hair, changes in color and texture of the hair, lethargy, and profound gonadotoxicity. Other side effects may include easy bruising/bleeding, joint pain, mouth sores, slow-healing existing wounds, unusual decrease in the amount of urine or unusual tiredness or weakness.[citation needed] Potential side effects also include leukopenia, infection, bladder toxicity, and cancer.[20]
Pulmonary injury appears rare,[21] but can present with two clinical patterns: an early, acute pneumonitis and a chronic, progressive fibrosis.[22] Cardiotoxicity is a major problem with people treated with higher dose regimens.[23]
High-dose intravenous cyclophosphamide can cause the syndrome of inappropriate antidiuretic hormone secretion (SIADH) and a potentially fatal hyponatremia when compounded by intravenous fluids administered to prevent drug-induced cystitis.[24] While SIADH has been described primarily with higher doses of cyclophosphamide, it can also occur with the lower doses used in the management of inflammatory disorders.[25]
Bladder bleeding
Acrolein is toxic to the bladder epithelium and can lead to hemorrhagic cystitis, which is associated with microscopic or gross hematuria and occasionally dysuria.[26] Risks of hemorrhagic cystitis can be minimized with adequate fluid intake, avoidance of nighttime dosage and mesna (sodium 2-mercaptoethane sulfonate), a sulfhydryl donor which binds and detoxifies acrolein.[27] Intermittent dosing of cyclophosphamide decreases cumulative drug dose, reduces bladder exposure to acrolein and has equal efficacy to daily treatment in the management of lupus nephritis.[28]
Infection
Neutropenia or lymphopenia arising secondary to cyclophosphamide usage can predispose people to a variety of bacterial, fungal and opportunistic infections.[29] No published guidelines cover PCP prophylaxis for people with rheumatological diseases receiving immunosuppressive drugs, but some advocate its use when receiving high-dose medication.[30][31]
Infertility
Cyclophosphamide has been found to significantly increase the risk of premature menopause in females and of infertility in males and females, the likelihood of which increases with cumulative drug dose and increasing patient age. Such infertility is usually temporary, but can be permanent.[32] The use of leuprorelin in women of reproductive age before administration of intermittently dosed cyclophosphamide may diminish the risks of premature menopause and infertility.[33]
Cancer
Cyclophosphamide is carcinogenic and may increase the risk of developing lymphomas, leukemia, skin cancer, transitional cell carcinoma of the bladder or other malignancies.[34] Myeloproliferative neoplasms, including acute leukemia, non-Hodgkin lymphoma and multiple myeloma, occurred in 5 of 119 rheumatoid arthritis patients within the first decade after receiving cyclophosphamide, compared with one case of chronic lymphocytic leukemia in 119 rheumatoid arthritis patients with no history.[35] Secondary acute myeloid leukemia (therapy-related AML, or "t-AML") is thought to occur either by cyclophosphamide-inducing mutations or selecting for a high-risk myeloid clone.[36]
This risk may be dependent on dose and other factors, including the condition, other agents or treatment modalities (including radiotherapy), treatment length and intensity. For some regimens, it is rare. For instance, CMF-therapy for breast cancer (where the cumulative dose is typically less than 20 grams of cyclophosphamide) carries an AML risk of less than 1/2000, with some studies finding no increased risk compared to background. Other treatment regimens involving higher doses may carry risks of 1–2% or higher.
Cyclophosphamide-induced AML, when it happens, typically presents some years after treatment, with incidence peaking around 3–9 years. After nine years, the risk falls to background. When AML occurs, it is often preceded by a myelodysplastic syndrome phase, before developing into overt acute leukemia. Cyclophosphamide-induced leukemia will often involve complex cytogenetics, which carries a worse prognosis than de novo AML.[citation needed]
Pharmacology
Oral cyclophosphamide is rapidly absorbed and then converted by mixed-function oxidase enzymes (cytochrome P450 system) in the liver to active metabolites.[37][38] The main active metabolite is 4-hydroxycyclophosphamide, which exists in equilibrium with its tautomer, aldophosphamide. Most of the aldophosphamide is then oxidised by the enzyme aldehyde dehydrogenase (ALDH) to make carboxycyclophosphamide. A small proportion of aldophosphamide freely diffuses into cells, where it is decomposed into two compounds, phosphoramide mustard and acrolein.[39] The active metabolites of cyclophosphamide are highly protein bound and distributed to all tissues, are assumed to cross the placenta and are known to be present in breast milk.[40]
It is specifically in the oxazaphosphorine group of medications.[41]
Cyclophosphamide metabolites are primarily excreted in the urine unchanged, and drug dosing should be appropriately adjusted in the setting of renal dysfunction.[42] Drugs altering hepatic microsomal enzyme activity (e.g., alcohol, barbiturates, rifampicin, or phenytoin) may result in accelerated metabolism of cyclophosphamide into its active metabolites, increasing both pharmacologic and toxic effects of the drug; alternatively, drugs that inhibit hepatic microsomal enzymes (e.g. corticosteroids, tricyclic antidepressants, or allopurinol) result in slower conversion of cyclophosphamide into its metabolites and consequently reduced therapeutic and toxic effects.[43]
Cyclophosphamide reduces plasma pseudocholinesterase activity and may result in prolonged neuromuscular blockade when administered concurrently with succinylcholine.[44][45] Tricyclic antidepressants and other anticholinergic agents can result in delayed bladder emptying and prolonged bladder exposure to acrolein.[citation needed]
Mechanism of action
The main effect of cyclophosphamide is due to its metabolite phosphoramide mustard. This metabolite is only formed in cells that have low levels of ALDH. Phosphoramide mustard forms DNA crosslinks both between and within DNA strands at guanine N-7 positions (known as interstrand and intrastrand crosslinkages, respectively). This is irreversible and leads to cell apoptosis.[46]
Cyclophosphamide has relatively little typical chemotherapy toxicity as ALDHs are present in relatively large concentrations in bone marrow stem cells, liver and intestinal epithelium. ALDHs protect these actively proliferating tissues against toxic effects of phosphoramide mustard and acrolein by converting aldophosphamide to carboxycyclophosphamide that does not give rise to the toxic metabolites phosphoramide mustard and acrolein. This is because carboxycyclophosphamide cannot undergo β-elimination (the carboxylate acts as an electron-donating group, nullifying the potential for transformation), preventing nitrogen mustard activation and subsequent alkylation.[26][47][48]
Cyclophosphamide induces beneficial immunomodulatory effects in adaptive immunotherapy. Suggested mechanisms include:[49]
- Elimination of T regulatory cells (CD4+CD25+ T cells) in naive and tumor-bearing hosts
- Induction of T cell growth factors, such as type I IFNs, and/or
- Enhanced grafting of adoptively transferred, tumor-reactive effector T cells by the creation of an immunologic space niche.
Thus, cyclophosphamide preconditioning of recipient hosts (for donor T cells) has been used to enhance immunity in naïve hosts, and to enhance adoptive T cell immunotherapy regimens, as well as active vaccination strategies, inducing objective antitumor immunity.
History
As reported by O. M. Colvin in his study of the development of cyclophosphamide and its clinical applications,
Phosphoramide mustard, one of the principal toxic metabolites of cyclophosphamide, was synthesized and reported by Friedman and Seligman in 1954[50] …It was postulated that the presence of the phosphate bond to the nitrogen atom could inactivate the nitrogen mustard moiety, but the phosphate bond would be cleaved in gastric cancers and other tumors which had a high phosphamidase content. However, in studies carried out after the clinical efficacy of cyclophosphamide was demonstrated, phosphoramide mustard proved to be cytotoxic in vitro (footnote omitted), but to have a low therapeutic index in vivo.[51]
Cyclophosphamide and the related nitrogen mustard–derived alkylating agent ifosfamide were developed by Norbert Brock and ASTA (now Baxter Oncology).[52] Brock and his team synthesised and screened more than 1,000 candidate oxazaphosphorine compounds.[53] They converted the base nitrogen mustard into a nontoxic "transport form". This transport form was a prodrug, subsequently actively transported into cancer cells. Once in the cells, the prodrug was enzymatically converted into the active, toxic form. The first clinical trials were published at the end of the 1950s.[54][55][56] In 1959 it became the eighth cytotoxic anticancer agent to be approved by the FDA.[26]
Society and culture
The abbreviation CP is common, although abbreviating drug names is not best practice in medicine.[57]
Research
Because of its impact on the immune system, it is used in animal studies. Rodents are injected intraperitoneally with either a single dose of 150 mg/kg or two doses (150 and 100 mg/kg) spread over two days.[58] This can be used for applications such as:
- The EPA may be concerned about potential human pathogenicity of an engineered microbe when conducting an MCAN review. Particularly for bacteria with potential consumer exposure they require testing of the microbe on immuno-compromised rats.[59]
- Cyclophosphamide provides a positive control when studying immune-response of a new drug.[60]
References
- ↑ "cyclophosphamide – definition of cyclophosphamide in English from the Oxford dictionary". OxfordDictionaries.com. https://www.oxforddictionaries.com/definition/english/cyclophosphamide.
- ↑ "Cyclophosphamide". Merriam-Webster Dictionary. https://www.merriam-webster.com/dictionary/cyclophosphamide.
- ↑ "NCI Drug Dictionary". 2 February 2011. http://www.cancer.gov/drugdictionary/?CdrID=39748.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 4.6 4.7 4.8 4.9 "Cyclophosphamide". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/cyclophosphamide.html.
- ↑ 5.0 5.1 "Updates in ANCA-associated vasculitis". European Journal of Rheumatology 3 (3): 122–133. September 2016. doi:10.5152/eurjrheum.2015.0043. PMID 27733943.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ 7.0 7.1 7.2 Brayfield, A, ed (9 January 2017). "Cyclophosphamide: Martindale: The Complete Drug Reference". London, UK: Pharmaceutical Press. https://www.medicinescomplete.com/mc/martindale/current/1825-k.htm.
- ↑ Brenner & Rector's The Kidney (11th ed.). Philadelphia: Elsevier. 2020. pp. 1007–1091. ISBN 978-0-323-53265-5.
- ↑ "Goodpasture Syndrome (Anti-glomerular Basement Membrane Antibody Disease)". StatPearls. Treasure Island, USA: StatPearls Publishing. 2019. https://www.ncbi.nlm.nih.gov/books/NBK459291/.
- ↑ "Cyclophosphamide for multiple sclerosis". The Cochrane Database of Systematic Reviews 2007 (1): CD002819. January 2007. doi:10.1002/14651858.CD002819.pub2. PMID 17253481.
- ↑ "Research and therapeutics-traditional and emerging therapies in systemic lupus erythematosus". Rheumatology 56 (suppl_1): i100–i113. April 2017. doi:10.1093/rheumatology/kew417. PMID 28375452.
- ↑ "Comparative effectiveness of immunosuppressive drugs and corticosteroids for lupus nephritis: a systematic review and network meta-analysis". Systematic Reviews 5 (1): 155. September 2016. doi:10.1186/s13643-016-0328-z. PMID 27619512.
- ↑ "Immunoglobulin light chain amyloidosis: 2014 update on diagnosis, prognosis, and treatment". American Journal of Hematology 89 (12): 1132–40. December 2014. doi:10.1002/ajh.23828. PMID 25407896.
- ↑ "The evolution of T-cell depletion in haploidentical stem-cell transplantation". British Journal of Haematology 172 (5): 667–84. March 2016. doi:10.1111/bjh.13868. PMID 26684279.
- ↑ "HLA-haploidentical blood or marrow transplantation with high-dose, post-transplantation cyclophosphamide". Bone Marrow Transplantation 50 (Suppl 2): S31–6. June 2015. doi:10.1038/bmt.2015.92. PMID 26039204.
- ↑ "Haploidentical bone marrow and stem cell transplantation: experience with post-transplantation cyclophosphamide". Seminars in Hematology 53 (2): 90–7. April 2016. doi:10.1053/j.seminhematol.2016.01.005. PMID 27000732.
- ↑ "Apparent cyclophosphamide (cytoxan) embryopathy: a distinct phenotype?". American Journal of Medical Genetics 86 (3): 237–41. September 1999. doi:10.1002/(SICI)1096-8628(19990917)86:3<237::AID-AJMG8>3.0.CO;2-V. PMID 10482872.
- ↑ "Toxicity profiles of disease modifying antirheumatic drugs in rheumatoid arthritis". The Journal of Rheumatology 18 (2): 188–94. February 1991. PMID 1673721.
- ↑ "The problem of permanent bone marrow damage after cytotoxic drug treatment". Oncology 41 (3): 180–4. 1984. doi:10.1159/000225819. PMID 6374556.
- ↑ "Risk of serious infections with immunosuppressive drugs and glucocorticoids for lupus nephritis: a systematic review and network meta-analysis". BMC Medicine 14 (1): 137. September 2016. doi:10.1186/s12916-016-0673-8. PMID 27623861.
- ↑ "Pulmonary effects of cytotoxic agents other than bleomycin". Clinics in Chest Medicine 11 (1): 31–54. March 1990. doi:10.1016/S0272-5231(21)00670-5. PMID 1691069.
- ↑ "Lung toxicity associated with cyclophosphamide use. Two distinct patterns". American Journal of Respiratory and Critical Care Medicine 154 (6 Pt 1): 1851–6. December 1996. doi:10.1164/ajrccm.154.6.8970380. PMID 8970380.
- ↑ "Cardiotoxicity of cancer therapy". Journal of Clinical Oncology 23 (30): 7685–96. October 2005. doi:10.1200/JCO.2005.08.789. PMID 16234530.
- ↑ "Water intoxication following moderate-dose intravenous cyclophosphamide". Archives of Internal Medicine 145 (3): 548–9. March 1985. doi:10.1001/archinte.145.3.548. PMID 3977522.
- ↑ "Water intoxication induced by low-dose cyclophosphamide in two patients with systemic lupus erythematosus". Lupus 12 (8): 636–9. 2003. doi:10.1191/0961203303lu421cr. PMID 12945725.
- ↑ 26.0 26.1 26.2 "Cyclophosphamide and cancer: golden anniversary". Nature Reviews. Clinical Oncology 6 (11): 638–47. November 2009. doi:10.1038/nrclinonc.2009.146. PMID 19786984.
- ↑ "Incidence and prevention of bladder toxicity from cyclophosphamide in the treatment of rheumatic diseases: a data-driven review". Arthritis and Rheumatism 62 (1): 9–21. January 2010. doi:10.1002/art.25061. PMID 20039416.
- ↑ "Controlled trial of pulse methylprednisolone versus two regimens of pulse cyclophosphamide in severe lupus nephritis". Lancet 340 (8822): 741–5. September 1992. doi:10.1016/0140-6736(92)92292-n. PMID 1356175. https://zenodo.org/record/1258313.
- ↑ "Risk factors for serious infection during treatment with cyclophosphamide and high-dose corticosteroids for systemic lupus erythematosus". Arthritis and Rheumatism 39 (9): 1475–82. September 1996. doi:10.1002/art.1780390906. PMID 8814058.
- ↑ "When is it safe to stop Pneumocystis jiroveci pneumonia prophylaxis? Insights from three cases complicating autoimmune diseases". Arthritis and Rheumatism 59 (7): 1034–9. July 2008. doi:10.1002/art.23822. PMID 18576286.
- ↑ "Frequency, risk factors and prophylaxis of infection in ANCA-associated vasculitis". European Journal of Clinical Investigation 45 (3): 346–68. March 2015. doi:10.1111/eci.12410. PMID 25627555.
- ↑ "NIH conference. Lupus nephritis". Annals of Internal Medicine 106 (1): 79–94. January 1987. doi:10.7326/0003-4819-106-1-79. PMID 3789582.
- ↑ "Clinical pharmacokinetics of depot leuprorelin". Clinical Pharmacokinetics 41 (7): 485–504. 2002. doi:10.2165/00003088-200241070-00003. PMID 12083977.
- ↑ "Hematologic malignant neoplasms after drug exposure in rheumatoid arthritis". Archives of Internal Medicine 168 (4): 378–81. February 2008. doi:10.1001/archinternmed.2007.107. PMID 18299492.
- ↑ "Effects of cyclophosphamide on the development of malignancy and on long-term survival of patients with rheumatoid arthritis. A 20-year followup study". Arthritis and Rheumatism 38 (8): 1120–7. August 1995. doi:10.1002/art.1780380815. PMID 7639809.
- ↑ "Etiology and management of therapy-related myeloid leukemia". Hematology. American Society of Hematology. Education Program 2007: 453–9. 2007. doi:10.1182/asheducation-2007.1.453. PMID 18024664.
- ↑ "Enzymatic basis of cyclophosphamide activation by hepatic microsomes of the rat". The Journal of Pharmacology and Experimental Therapeutics 174 (2): 206–10. August 1970. PMID 4393764. http://jpet.aspetjournals.org/cgi/pmidlookup?view=long&pmid=4393764. Retrieved 2014-05-02.
- ↑ "Prodrugs--from serendipity to rational design". Pharmacological Reviews 63 (3): 750–71. September 2011. doi:10.1124/pr.110.003459. PMID 21737530.
- ↑ "Metabolism and pharmacokinetics of oxazaphosphorines". Clinical Pharmacokinetics 38 (4): 291–304. April 2000. doi:10.2165/00003088-200038040-00001. PMID 10803453.
- ↑ "Cyclophosphamide in human milk". Lancet 1 (7705): 912. May 1971. doi:10.1016/s0140-6736(71)92474-3. PMID 4102054.
- ↑ "Oxazaphosphorines: new therapeutic strategies for an old class of drugs". Expert Opinion on Drug Metabolism & Toxicology 6 (8): 919–938. August 2010. doi:10.1517/17425255.2010.487861. PMID 20446865.
- ↑ "Cyclophosphamide pharmacokinetics and dose requirements in patients with renal insufficiency". Kidney International 61 (4): 1495–501. April 2002. doi:10.1046/j.1523-1755.2002.00279.x. PMID 11918757.
- ↑ "Importance of pharmacokinetic studies on cyclophosphamide (NSC-26271) in understanding its cytotoxic effect". Cancer Treatment Reports 60 (4): 395–401. April 1976. PMID 1277213.
- ↑ "Acquired pseudocholinesterase deficiency after high-dose cyclophosphamide". Bone Marrow Transplantation 24 (12): 1367–8. December 1999. doi:10.1038/sj.bmt.1702097. PMID 10627651.
- ↑ "[Prolonged neuromuscular block induced by mivacurium in a patient treated with cyclophosphamide]" (in fr). Annales Françaises d'Anesthésie et de Réanimation 14 (6): 508–10. 1995. doi:10.1016/S0750-7658(05)80493-9. INIST:2947795. PMID 8745976.
- ↑ "Mechanisms of action of, and modes of resistance to, alkylating agents used in the treatment of haematological malignancies". Blood Reviews 6 (3): 163–73. September 1992. doi:10.1016/0268-960X(92)90028-O. PMID 1422285.
- ↑ "Aldehyde dehydrogenase activity as the basis for the relative insensitivity of murine pluripotent hematopoietic stem cells to oxazaphosphorines". Biochemical Pharmacology 34 (19): 3465–71. October 1985. doi:10.1016/0006-2952(85)90719-1. PMID 2996550.
- ↑ "Cyclophosphamide (NSC-26271)-related phosphoramide mustards- recent advances and historical perspective". Cancer Treatment Reports 60 (4): 337–46. April 1976. PMID 1277209.
- ↑ "Immunomodulatory effects of cyclophosphamide and implementations for vaccine design". Seminars in Immunopathology 33 (4): 369–83. July 2011. doi:10.1007/s00281-011-0245-0. PMID 21611872.
- ↑ "Preparation of N-Phosphorylated Derivatives of Bis-β-chloroethylamine1a". Journal of the American Chemical Society 76 (3): 655–8. 1954. doi:10.1021/ja01632a006.
- ↑ "An overview of cyclophosphamide development and clinical applications". Current Pharmaceutical Design 5 (8): 555–60. August 1999. doi:10.2174/1381612805666230110214512. PMID 10469891.
- ↑ U.S. Patent 3,018,302
- ↑ "The history of the oxazaphosphorine cytostatics". Cancer 78 (3): 542–7. August 1996. doi:10.1002/(SICI)1097-0142(19960801)78:3<542::AID-CNCR23>3.0.CO;2-Y. PMID 8697402.
- ↑ (in de) Chemotherapie maligner Tumoren. Asta-Forschung und Therapie. 1958. OCLC 73296245.[page needed]
- ↑ "Klinische und experimentelle Erfahrungen mit zyk lischen und nichtzyklischen Phosphamidestern des N-Losl in der Chemotherapie von Tumoren" (in de). Strahlentherapie 41: 361–7. 1959.
- ↑ "Oxazaphosphorine cytostatics: past-present-future. Seventh Cain Memorial Award lecture". Cancer Research 49 (1): 1–7. January 1989. PMID 2491747. http://cancerres.aacrjournals.org/cgi/pmidlookup?view=long&pmid=2491747.
- ↑ Institute for Safe Medication Practices, ISMP's List of Error-Prone Abbreviations, Symbols, and Dose Designations, http://www.ismp.org/tools/errorproneabbreviations.pdf.
- ↑ "Neutropenia induced in outbred mice by a simplified low-dose cyclophosphamide regimen: characterization and applicability to diverse experimental models of infectious diseases" (in En). BMC Infectious Diseases 6 (1): 55. March 2006. doi:10.1186/1471-2334-6-55. PMID 16545113.
- ↑ "EPA: Notifications, FY 1998 to Present - Biotechnology Program under the Toxic Substances Control Act (TSCA) | New Chemicals Program | US EPA". http://www.epa.gov/biotech_rule/pubs/submiss.htm.
- ↑ "Immunosuppressive effect of cyclophosphamide on white blood cells and lymphocyte subpopulations from peripheral blood of Balb/c mice". International Immunopharmacology 11 (9): 1293–7. September 2011. doi:10.1016/j.intimp.2011.04.011. PMID 21530682. https://zenodo.org/record/895552.
External links
- "Cyclophosphamide". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/Cyclophosphamide+(anhydrous).
- U.S. Patent 3,018,302 Novel cyclic phosphoric acid ester amides, and the production thereof. (patent for cyclophosphamide).
 |