Chemistry:SAGE-718
From HandWiki
Short description: Chemical compound
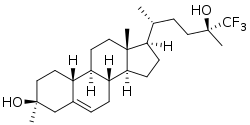 | |
| Legal status | |
|---|---|
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| Chemical and physical data | |
| Formula | C26H41F3O2 |
| Molar mass | 442.607 g·mol−1 |
SAGE-718 is experimental drug being investigated for the treatment of neurological disorders and cognitive impairment.[1] It acts as a positive allosteric modulator of the NMDA receptor, whose activity is essential for learning, memory, and cognition.[2] SAGE-718 is an analog of the neurosteroid 24S-hydroxycholesterol.[2]
As of 2022, SAGE-718 is in Phase II clinical trials[2] for Alzheimer's disease,[3][4][5] Parkinson's disease, and Huntington's disease.[6][7]
References
- ↑ "SAGE-718: A First-in-Class N-Methyl-d-Aspartate Receptor Positive Allosteric Modulator for the Potential Treatment of Cognitive Impairment". Journal of Medicinal Chemistry. July 2022. doi:10.1021/acs.jmedchem.2c00313. PMID 35785990.
- ↑ 2.0 2.1 2.2 "SAGE-718". ALZFORUM. https://www.alzforum.org/therapeutics/sage-718.
- ↑ "#AAN2022 – SAGE-718 May Help With Cognitive Function in Alzheimer's". BioNews, Inc.. https://alzheimersnewstoday.com/news/aan-2022-sage-718-well-tolerated-help-cognitive-function-patients-mild-impairments/.
- ↑ "SAGE-718 in Patients With Mild Cognitive Impairment or Mild Dementia Due to Alzheimer's Disease: Results From the Phase 2 LUMINARY Study". American Academy of Neurology (AAN) 74th Annual Meeting Abstract. https://aanfiles.blob.core.windows.net/aanfiles/ca8704ff-7709-43fd-8f8a-9a08a8c137bb/EMBARGOED%202022%20AAN%20AM%20Abstract%20%231136%20-%20SAGE-718%20in%20Patients%20With%20Mild%20Cognitive%20Impairment%20or%20Mild%20Dementia%20due%20to%20Alzheimer's%20-%20(1).pdf.
- ↑ "Regulatory roundup: Sage Alzheimer's asset likely to progress after Phase IIa completion". Clinical Trials Arena. 16 November 2021. https://www.clinicaltrialsarena.com/analysis/sage-therapeutics-alzheimers-disease-regulatory-roundup/.
- ↑ "Sage Therapeutics Provides Important Update On The Clinical Development Program For Sage's Investigational Drug, SAGE-718.". Huntington's Disease Society of America. https://hdsa.org/news/sage-therapeutics-provides-important-update-on-the-clinical-development-program-for-sages-investigational-drug-sage-718/.
- ↑ "SAGE-718 on FDA Fast Track as Potential Huntington's Disease Therapy". BioNews, Inc.. 21 September 2021. https://huntingtonsdiseasenews.com/news/sage-718-on-fda-fast-track-potential-huntingtons-therapy/.

