Chemistry:Trazpiroben
From HandWiki
Short description: Chemical compound
 | |
| Clinical data | |
|---|---|
| Other names | TAK-906; ATC-1906 |
| Drug class | Dopamine antagonist |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| Chemical and physical data | |
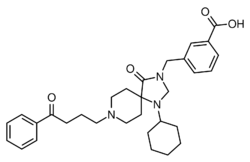
| Formula | C31H39N3O4 |
| Molar mass | 517.670 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Trazpiroben (developmental code name TAK-906) is a dopamine antagonist drug which was under development for the treatment of gastroparesis.[1][2][3][4][5] It acts as a peripherally selective dopamine D2 and D3 receptor antagonist.[1][2] The drug has been found to strongly increase prolactin levels in humans, similarly to other peripherally selective D2 receptor antagonists like domperidone.[5] Clinical development of trazpiroben was discontinued before April 2022.[1] Trazpiroben was originated by Altos Therapeutics and was under development by Takeda Oncology.[1]
References
- ↑ 1.0 1.1 1.2 1.3 "Trazpiroben - Takeda Oncology - AdisInsight". https://adisinsight.springer.com/drugs/800049598.
- ↑ 2.0 2.1 "Preclinical Evaluation of the Effects of Trazpiroben (TAK-906), a Novel, Potent Dopamine D2/D3 Receptor Antagonist for the Management of Gastroparesis". J Pharmacol Exp Ther 379 (1): 85–95. October 2021. doi:10.1124/jpet.121.000698. PMID 34253646.
- ↑ "Safety, Pharmacokinetics, and Pharmacodynamics of Trazpiroben (TAK-906), a Novel Selective D2 /D3 Receptor Antagonist: A Phase 1 Randomized, Placebo-Controlled Single- and Multiple-Dose Escalation Study in Healthy Participants". Clin Pharmacol Drug Dev 10 (8): 927–939. August 2021. doi:10.1002/cpdd.906. PMID 33462988.
- ↑ "Randomised clinical trial: safety, pharmacokinetics and pharmacodynamics of trazpiroben (TAK-906), a dopamine D2 /D3 receptor antagonist, in patients with gastroparesis". Aliment Pharmacol Ther 54 (3): 267–280. August 2021. doi:10.1111/apt.16451. PMID 34148244.
- ↑ 5.0 5.1 "Evaluating the Safety, Tolerability, and Disposition of Trazpiroben, a D2 /D3 Receptor Antagonist: Phase I Single- and Multiple-Ascending Dose Studies in Healthy Japanese Participants". Clin Pharmacol Drug Dev 11 (6): 695–706. December 2021. doi:10.1002/cpdd.1057. PMID 34967147.
External links
 |
