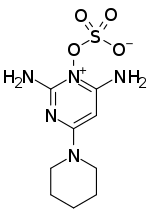
Chemistry:Minoxidil sulfate
 | |
| Clinical data | |
|---|---|
| Other names | Minoxidil sulphate; Minoxidil sulfate ester; Minoxidil sulphate ester; Minoxidil N-O-sulfate; Minoxidil N-O-sulphate; U-58838 |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C9H15N5O4S |
| Molar mass | 289.31 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
Minoxidil sulfate, also known as minoxidil sulfate ester or minoxidil N-O-sulfate, is an active metabolite of minoxidil (Rogaine, Loniten, others) and is the active form of this agent.[1][2] Minoxidil acts as a prodrug of minoxidil sulfate.[1] Minoxidil sulfate is formed from minoxidil via sulfotransferase enzymes, with the predominant enzyme responsible, at least in hair follicles, being SULT1A1.[1][2] Minoxidil sulfate acts as a potassium channel opener, among other actions, and has vasodilating, hypotensive, and trichogenic or hypertrichotic (hair growth-promoting) effects.[1][3] Its mechanism of action in terms of hair growth is still unknown, although multiple potential mechanisms have been implicated.[1]
Minoxidil sulfate is a sulfate ester of minoxidil, not a sulfate salt of the compound.[3] However, minoxidil sulfate forms an inner salt, which makes it more hydrophobic than minoxidil.[3] This is in contrast to most sulfate esters, which are usually more hydrophilic than their non-ester forms.[3] The bioactivation of minoxidil into minoxidil sulfate is very unusual and is among the only known instances of sulfation producing a more active drug form.[3][4] Normally, sulfation tends to inactivate drugs by reducing their biological activity and increasing their excretion.[3][4]
Minoxidil sulfate is highly unstable in aqueous solutions and alcohol-containing solvents, with a half-life of 6 hours in aqueous solutions and a further much lower half-life in alcohol-containing solvents.[3] This has served as a limiting factor in its potential pharmaceutical use and therapeutic effectiveness.[5] Moreover, minoxidil sulfate has a 40% higher molecular weight than minoxidil, and this may reduce its absorption into the scalp.[5] In any case, a minoxidil sulfate-based topical formulation has been investigated for the treatment of scalp hair loss.[6][5] Additionally, minoxidil-sulfate-based topical formulations appear to be available for medical use in some parts of the world, for instance in Brazil.[5][7]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 "Minoxidil: a comprehensive review". The Journal of Dermatological Treatment 33 (4): 1896–1906. June 2022. doi:10.1080/09546634.2021.1945527. PMID 34159872.
- ↑ 2.0 2.1 "Review of oral minoxidil as treatment of hair disorders: in search of the perfect dose". Journal of the European Academy of Dermatology and Venereology 35 (7): 1485–1492. July 2021. doi:10.1111/jdv.17216. PMID 33660357.
- ↑ 3.0 3.1 3.2 3.3 3.4 3.5 3.6 "Enzymatic and non-enzymatic sulfation mechanisms in the biological actions of minoxidil". Biochemical Pharmacology 45 (2): 271–279. January 1993. doi:10.1016/0006-2952(93)90061-z. PMID 8435087.
- ↑ 4.0 4.1 Richard B. Silverman (2 December 2012). The Organic Chemistry of Drug Design and Drug Action (2 ed.). Elsevier. pp. 543–. ISBN 978-0-08-051337-9. OCLC 1019583017. https://books.google.com/books?id=DKuDExo905UC&pg=PA543.
- ↑ 5.0 5.1 5.2 5.3 "Use of Minoxidil Sulfate versus Minoxidil Base in Androgenetic Alopecia Treatment: Friend or Foe?". Skin Appendage Disorders 4 (4): 349–350. October 2018. doi:10.1159/000488011. PMID 30410915.
- ↑ "Minoxidil and its use in hair disorders: a review". Drug Design, Development and Therapy 13: 2777–2786. 2019. doi:10.2147/DDDT.S214907. PMID 31496654.
- ↑ "Androgenetic Alopecia: Clinical Treatment". Hair and Scalp Treatments. Springer International Publishing. 13 September 2019. pp. 91–108. doi:10.1007/978-3-030-21555-2_8. ISBN 978-3-030-21554-5.
 |

