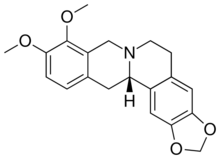
Chemistry:Canadine
| |
| Names | |
|---|---|
| IUPAC name
9,10-Dimethoxy-2′H-[1,3]dioxolo[4′,5′:2,3]berbine
| |
| Systematic IUPAC name
(13aS)-9,10-Dimethoxy-5,8,13,13a-tetrahydro-2H,6H-[1,3]dioxolo[4,5-g]isoquinolino[3,2-a]isoquinoline | |
| Other names
(S)-Tetrahydroberberine; Xanthopuccine
| |
| Identifiers | |
3D model (JSmol)
|
|
| ChEMBL | |
| ChemSpider | |
| KEGG | |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| C20H21NO4 | |
| Molar mass | 339.391 g·mol−1 |
Except where otherwise noted, data are given for materials in their standard state (at 25 °C [77 °F], 100 kPa). | |
| Infobox references | |
(S)-Canadine, also known as (S)-tetrahydroberberine and xanthopuccine, is a benzylisoquinoline alkaloid (BIA), of the protoberberine structural subgroup, and is present in many plants from the family Papaveraceae, such as Corydalis yanhusuo and C. turtschaninovii.
Biosynthesis
Metabolically, (S)-canadine is derived from (S)-reticuline, a pivotal intermediate in the biosynthesis of numerous BIA structural subgroups, through three enzymatic steps: 1) Berberine bridge enzyme to (S)-scoulerine; 2) (S)-scoulerine 9-O-methyltransferase to (S)-tetrahydrocolumbamine; and 3) (S)-canadine synthase/CYP719A21 to (S)-canadine.[1]
(S)-Canadine is the immediate metabolic precursor of berberine, which is obtained through the action of the enzyme (S)-tetrahydroprotoberberine oxidase.[1] It is also an intermediate in the complex biosynthesis of noscapine, which is likewise a benzylisoquinoline alkaloid, but of the phthalideisoquinoline structural subgroup.[2][3]
(S)-Canadine, berberine, palmatine, and hydrastine are the major alkaloids present in goldenseal.
Effects
A number of in vitro effects of (S)-canadine have been reported. It stimulates myogenesis and inhibits muscle protein degradation.[4] (S)-Canadine blocks K(ATP) channels in dopamine neurons.[5][6] (S)-Canadine has displayed antioxidant activity: though it lacked any demonstrable cytotoxic effect in three unique cell cultures, it was observed to possess antioxidant activity against free radical-induced oxidative injury.[7][8] (S)-Canadine can block voltage-dependent calcium channels, but at a level significantly lower than that of verapamil.[9]
References
- ↑ 1.0 1.1 Hagel, Jillian M.; Morris, Jeremy S.; Lee, Eun-Jeong; Desgagne-Penix, Isabel; Bross, Crystal D.; Chang, Limei; Chen, Xue; Farrow, Scott C. et al. (2015). "Transcriptome analysis of 20 taxonomically related benzylisoquinoline alkaloid-producing plants". BMC Plant Biology 15: 227. doi:10.1186/s12870-015-0596-0. PMID 26384972.
- ↑ Dang, Thu-Thuy T.; Facchini, Peter J. (2014). "CYP82Y1 is N-Methylcanadine 1-Hydroxylase, a Key Noscapine Biosynthetic Enzyme in Opium Poppy". The Journal of Biological Chemistry 289 (4): 2013–2026. doi:10.1074/jbc.M113.505099. PMID 24324259. PMC 3900951. http://www.jbc.org/content/289/4/2013.full.pdf.
- ↑ Chen, Xue; Facchini, Peter J. (2014). "Short-chain dehydrogenase/reductase catalyzing the final step of noscapine biosynthesis is localized to laticifers in opium poppy". The Plant Journal 77 (2): 173–184. doi:10.1111/tpj.12379. PMID 24708518.
- ↑ Lee, Hyejin; Lee, Sang-Jin; Bae, Gyu-Un; Baek, Nam-In; Ryu, Jae-Ha (2017). "Canadine from Corydalis turtschaninovii Stimulates Myoblast Differentiation and Protects against Myotube Atrophy". International Journal of Molecular Sciences 18 (12): 2748. doi:10.3390/ijms18122748. PMID 29258243.
- ↑ Wu, Chen; Yang, Kechun; Liu, Qiang; Wakui, Matoko; Jin, Guo-zhang; Zhen, Xuechu; Wu, Jie (2010). "Tetrahydroberberine blocks ATP-sensitive potassium channels in dopamine neurons acutely-dissociated from rat substantia nigra pars compacta". Neuropharmacology 59 (7–8): 567–72. doi:10.1016/j.neuropharm.2010.08.018. PMID 20804776.
- ↑ Wu, Jie; Jin, Guo Zhang (1997). "Tetrahydroberberine blocks membrane K+ channels underlying its inhibition of intracellular message-mediated outward currents in acutely dissociated CA1 neurons from rat hippocampus". Brain Research 775 (1–2): 214–8. doi:10.1016/s0006-8993(97)00960-8. PMID 9439847.
- ↑ Correché, Estela R.; Andujar, Sebastian A.; Kurdelas, Rita R.; Lechón, María J. Gómez; Freile, Mónica L.; Enriz, Ricardo D. (2008). "Antioxidant and cytotoxic activities of canadine: Biological effects and structural aspects". Bioorganic & Medicinal Chemistry 16 (7): 3641–51. doi:10.1016/j.bmc.2008.02.015. PMID 18295494.
- ↑ Mari, Giacomo; Catalani, Simona; Antonini, Elena; De Crescentini, Lucia; Mantellini, Fabio; Santeusanio, Stefania; Lombardi, Paolo; Amicucci, Antonella et al. (2018). "Synthesis and biological evaluation of novel heteroring-annulated pyrrolino-tetrahydroberberine analogues as antioxidant agents". Bioorganic & Medicinal Chemistry 26 (18): 5037–44. doi:10.1016/j.bmc.2018.08.038. PMID 30196978.
- ↑ Yang, S; Miao, Y.S.; Han, Q; Jiang, M.H.; Jin, G.Z. (1993). "Effects of (-)-stepholidine and tetrahydroberberine on high potassium-evoked contraction and calcium influx in rat artery". Zhongguo Yao Li Xue Bao 14 (3): 235–7. PMID 8237399. http://www.chinaphar.com/article/view/8753/9357.
 |
