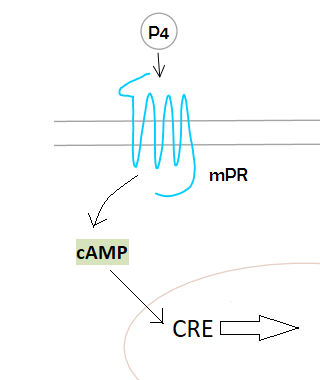
Biology:Membrane progesterone receptor
Membrane progesterone receptors (mPRs) are a group of cell surface receptors and membrane steroid receptors belonging to the progestin and adipoQ receptor (PAQR) family which bind the endogenous progestogen and neurosteroid progesterone, as well as the neurosteroid allopregnanolone.[1][2] Unlike the progesterone receptor (PR), a nuclear receptor which mediates its effects via genomic mechanisms, mPRs are cell surface receptors which rapidly alter cell signaling via modulation of intracellular signaling cascades.[1] The mPRs mediate important physiological functions in male and female reproductive tracts, liver, neuroendocrine tissues, and the immune system as well as in breast and ovarian cancer. The mPRs appear to be involved in the neuroprotective and antigonadotropic effects of progesterone and allopregnanolone.[1][2] The progesterone active metabolites 5α-dihydroprogesterone, also a progestogen, and allopregnanolone, which are positive allosteric modulators of the GABAA receptor, have been found to rapidly influence sexual receptivity and behavior in mice, actions that are GABAA receptor-dependent.[3][4]
These proteins are classified into three groups known as mPRα (PAQR7), mPRβ (PAQR8), mPRγ (PAQR5), mPRδ (PAQR6), and mPRϵ (PAQR9).
mPR Subtypes
mPRα
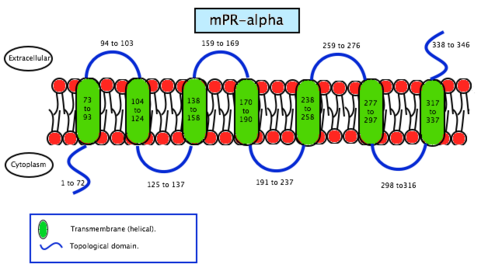
Membrane progesterone receptor alpha (mPRα) is a protein that in humans is encoded by the PAQR7 gene.[6] It is a steroid receptor which binds progesterone in vitro. Recent studies suggest the mPRα has important physiological functions in a variety of reproductive tissues. The mPRα is an intermediary in progestin induction of oocyte maturation and stimulation of sperm hyper motility in fish. In mammals, the mPRα has been implied in progesterone regulation of uterine functions in humans and GnRH secretion in rodents.[7]
mPRβ
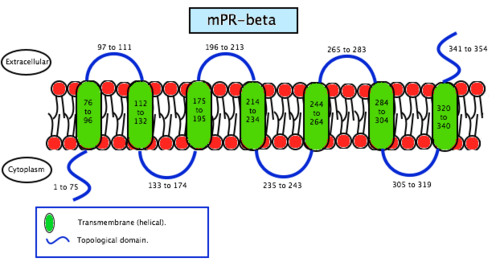
Membrane progesterone receptor beta (mPRβ) is a protein that in humans is encoded by the PAQR8 gene.[9]
A recent study[10] has investigated the role of mPRβ in regulating in vitro maturation (IVM) of pig cumulus-oocyte complexes (COCs). This study suggests that the mPRβ is a molecule related to cumulus expansion and it might function by regulation of exocytosis. The conclusion of this study is that mPRβ might play an important role on the function of the protein.
mPRγ
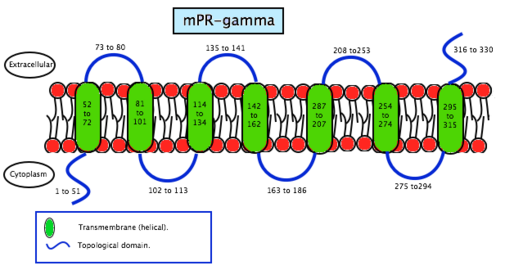
Membrane progesterone receptor gamma (mPRγ) is a protein that in humans is encoded by the PAQR5 gene.[12]
A study about the mPRγ subtype[13] has generated an antibody against this receptor in order to explore the role of mPRγ. Scientists found that mPRγ is expressed in female mouse reproductive tissues such as ovary and fallopian tube, and also in the lung and liver of both sexes. Immunohistochemical studies revealed that mPRγ is associated with the apical membrane of ciliated cells in the lumen of the fallopian tube, including human cells. That suggests a common role for mPRγ in the regulation of ciliary activity in the fallopian tube and the gamete transport in mammals. The presence of mPRγ in lung and liver of mice indicates that the receptor mediates the actions of progesterone outside the reproductive tract as well.
mPRδ
Membrane progesterone receptor delta (mPRδ) is a protein that in humans is encoded by the PAQR6 gene.[14]
mPRϵ
Membrane progesterone receptor epsilon (mPRϵ) is a protein that in humans is encoded by the PAQR9 gene.[15]
Summary table of features
Family members include:[16]
| mPR subtype | Gene | Length | Mass (Da) | Tissue specificity |
|---|---|---|---|---|
| mPRα | PAQR7 | 346 | 39,719 | Ovary, testis, placenta, uterus, bladder, others[17] |
| mPRβ | PAQR8 | 354 | 40,464 | Brain, spinal cord, kidney, testis, others[17] |
| mPRγ | PAQR5 | 330 | 38,014 | Brain, lung, kidney, colon, adrenal, others[17] |
| mPRδ | PAQR6 | 344 | 37,989 | Brain, breast, others[17][18] |
| mPRϵ | PAQR9 | 377 | 42,692 | Brain, breast, others[17][18] |
The general functions of these subtypes of mPR are: being steroid membrane receptors and binding progesterone. They also may be involved in oocyte maturation.
Potential roles
The discovery of a membrane located progesterone receptor (mPR) unrelated to the classical progesterone receptor (PR) in fish ovaries and its subsequent identification in mammal tissues suggests that mPRs could be a potential mediator of non-traditional progesterone actions, particularly in tissues where PR is absent. Even though classical PRs and mPRs can also have overlapping regional expression (e.g., both are expressed in the hippocampus, cortex, hypothalamus and cerebellum), their ligand specificity is not identical (for example mPRs bind to 17α-hydroxyprogesterone and 5-dihydroprogesterone with greater affinity than to the classical PRs).[19][20]
Many of progesterone's actions are too fast to be readily explained by a genomic mechanism which typically occurs over a time scale of hours – like most of the classical functions of progesterone mediated by progesterone receptors PR-A and PR-B, which mediate progesterone’s regulation of diverse female vertebrate reproductive functions through altering gene transcription – and it is now widely accepted that progesterone can also exert fast cell surface-initiated actions within minutes through activation of membrane receptors and their associated intracellular signaling paths.[1]
While some of the alternative progesterone actions are nongenomic, others may ultimately lead to altered gene transcription involving the activation of second messengers (such as MAP kinases) and through the alteration of progesterone receptors transactivation through effects on coactivators (such as SRC2).[1]
Extensive evidence has been obtained by different research groups that wild-type mPRs in a wide range of vertebrate cells as well as recombinant proteins expressed in prokaryotic and eukaryotic systems display high-affinity, specific, displaceable and limited capacity progesterone binding characteristic of steroid membrane receptors. Therefore, membrane progesterone receptors are good candidates for the membrane receptors mediating many of the nonclassical cell surface-initiated progesterone actions, such as oocyte meiotic maturation, granulosa cell apoptosis, immunosuppression of T cells, breast and ovarian cells.[citation needed]
It has been found that allopregnanolone, an effective mPR ligand, can act as an mPR agonist at low physiologically relevant concentrations. This indicates an additional receptor mechanism by which neurosteroids can potentially modulate neural functions.[1]
Experimental evidence also supports that mPRs are intermediaries in progestin-induced cell survival. MAP kinase and Akt are involved in inhibition of apoptosis, and it has been demonstrated that progestin activates MAP kinase and Akt through mPRs. This is a fact that consolidates mPR's antiapoptotic functions, and also their potential involvement in the antiapoptotic effects of allopregnanolone in the central nervous system.[1][21]
MPRs are also considered potential intermediaries in progesterone modulation of GnRH secretion under certain conditions, but direct evidence is lacking.
Structure
As the name says, mPRS are a group of proteins with a receptor function. This determines its location in the cell, the membrane. MPRs recognise some specific substances and facilitate the entrance of these substances inside compartments. Specifically, this receptors allow the entrance to the cell, therefore they are found in the plasmatic membrane. Studies have not revealed significant information about its structure so scientists still do not know exactly how this molecules are. In contrast, studies of the translated cDNAs based on the structure suggest they encode seven transmembrane domains. It also shows that mPRs have high affinity (Kd= 20-30 nm) saturable binding for progesterone – Kd is a constant of every enzyme which says the concentration of ligand needed in order to obtain the half of the saturation. Scientists went further on the study of the binding to the γ-subtype, revealing a specific binding for progesterone with a rate of association and dissociation of t1/2=2–8 minutes. MPRs have a molecular mass of approximately 40 kDa.[20] This results suggest that it may exist a new family of steroid receptors, also with the characteristics of G protein-coupled receptors. Another fact that suggests that this mPR subtype may be a G protein-coupled receptor is that it functions as the intermediary in progesterone induction of the maturation of oocyte meiotic maturation in teleost fishes.[22]
Involvement in cancer
Progesterone takes part in the growth regulation of different kind of tumors, in part by its interactions with its intracellular receptors (PR). MPRs have been found in cancer cells and tissues too. Their roles in the process are unclear but it has been suggested that, at least, this steroid hormone may inhibit tumor progression. Recently, it has been reported that membrane progesterone receptors (mPRs) are expressed in ovarian and breast cancer cells, and that progesterone could exert some actions through these receptors. The presence of functional mPRα, mPRβ, and mPRγ subtypes were detected in both cell lines as well as in breast tumor tissues.[23][24]
In the case of the ovarian cancer, transcripts for two of the three mPRs, α and β, were differentially expressed in ovarian cystadenomas, borderline tumors, and carcinomas: while mPRα is expressed at significantly higher levels than the others, an increased expression of mPRβ has been noticed in mucinous carcinomas when compared to the other tumor types and normal tissues. Notably, the expression of mPRγ was significantly higher in endometrioid and clear cell carcinomas, which are closely related tumors.[25] In one study, an increase in progesterone was shown to coincide with a reduced level of mPRγ and concomitant increase in the mPRα transcript levels.[26]
Recent studies suggest that some progesterone actions in astrocytoma cells (the most common and malignant human brain tumors) may be mediated also by mPRs. Recently, it has also been discovered that mPRα and mPRβ are clearly expressed at mRNA and protein levels in astrocytoma cells whereas mPRγ was barely expressed in these cells.[27]
See also
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5 1.6 "Membrane progesterone receptors: evidence for neuroprotective, neurosteroid signaling and neuroendocrine functions in neuronal cells". Neuroendocrinology 96 (2): 162–71. 2012. doi:10.1159/000339822. PMID 22687885.
- ↑ 2.0 2.1 "Novel progesterone receptors: neural localization and possible functions". Frontiers in Neuroscience 7: 164. 2013. doi:10.3389/fnins.2013.00164. PMID 24065878.
- ↑ "Neurobiology of sexual behavior". Current Opinion in Neurobiology 9 (6): 751–8. Dec 1999. doi:10.1016/s0959-4388(99)00034-3. PMID 10607643.
- ↑ "The neurosteroids, progesterone and 3alpha,5alpha-THP, enhance sexual motivation, receptivity, and proceptivity in female rats". Brain Research 808 (1): 72–83. Oct 1998. doi:10.1016/s0006-8993(98)00764-1. PMID 9795145.
- ↑ "PAQR7 - Membrane progestin receptor alpha - Homo sapiens (Human) - PAQR7 gene & protein". https://www.uniprot.org/uniprot/Q86WK9#subcellular_location.
- ↑ "PAQR7 - Membrane progestin receptor alpha - Homo sapiens (Human) - PAQR7 gene & protein". https://www.uniprot.org/uniprot/Q86WK9.
- ↑ "Characteristics of membrane progestin receptor alpha (mPRalpha) and progesterone membrane receptor component 1 (PGMRC1) and their roles in mediating rapid progestin actions". Frontiers in Neuroendocrinology 29 (2): 292–312. May 2008. doi:10.1016/j.yfrne.2008.01.001. PMID 18343488.
- ↑ "PAQR8 - Membrane progestin receptor beta - Homo sapiens (Human) - PAQR8 gene & protein". https://www.uniprot.org/uniprot/Q8TEZ7#subcellular_location.
- ↑ "PAQR8 - Membrane progestin receptor beta - Homo sapiens (Human) - PAQR8 gene & protein". https://www.uniprot.org/uniprot/Q8TEZ7.
- ↑ "Membrane progestin receptor beta (mPR-beta): a protein related to cumulus expansion that is involved in in vitro maturation of pig cumulus-oocyte complexes". Steroids 73 (14): 1416–23. Dec 2008. doi:10.1016/j.steroids.2008.07.007. PMID 18722396.
- ↑ "PAQR5 - Membrane progestin receptor gamma - Homo sapiens (Human) - PAQR5 gene & protein". https://www.uniprot.org/uniprot/Q9NXK6#subcellular_location.
- ↑ "PAQR5 - Membrane progestin receptor gamma - Homo sapiens (Human) - PAQR5 gene & protein". https://www.uniprot.org/uniprot/Q9NXK6#entry_information.
- ↑ "Membrane progesterone receptor gamma: tissue distribution and expression in ciliated cells in the fallopian tube". Molecular Reproduction and Development 74 (7): 843–50. Jul 2007. doi:10.1002/mrd.20685. PMID 17154304.
- ↑ "PAQR6 - Membrane progestin receptor delta - Homo sapiens (Human) - PAQR6 gene & protein". https://www.uniprot.org/uniprot/Q6TCH4#entry_information.
- ↑ "PAQR9 - Membrane progestin receptor epsilon - Homo sapiens (Human) - PAQR9 gene & protein". https://www.uniprot.org/uniprot/Q6ZVX9#entry_information.
- ↑ "membrane progestin receptor in UniProtKB". https://www.uniprot.org/uniprot/?query=membrane+progestin+receptor&sort=score.
- ↑ 17.0 17.1 17.2 17.3 17.4 "PAQR proteins: a novel membrane receptor family defined by an ancient 7-transmembrane pass motif". J. Mol. Evol. 61 (3): 372–80. 2005. doi:10.1007/s00239-004-0375-2. PMID 16044242. Bibcode: 2005JMolE..61..372T.
- ↑ 18.0 18.1 "Characterization, neurosteroid binding and brain distribution of human membrane progesterone receptors δ and {epsilon} (mPRδ and mPR{epsilon}) and mPRδ involvement in neurosteroid inhibition of apoptosis". Endocrinology 154 (1): 283–95. 2013. doi:10.1210/en.2012-1772. PMID 23161870.
- ↑ 19.0 19.1 "Membrane progesterone receptor expression in mammalian tissues: a review of regulation and physiological implications". Steroids 76 (1–2): 11–7. Jan 2011. doi:10.1016/j.steroids.2010.09.006. PMID 20869977.
- ↑ 20.0 20.1 "Non-genomic mechanisms of progesterone action in the brain". Frontiers in Neuroscience 7: 159. 2013-01-01. doi:10.3389/fnins.2013.00159. PMID 24065876.
- ↑ "The activation of Akt/PKB signaling pathway and cell survival". Journal of Cellular and Molecular Medicine 9 (1): 59–71. 2005-03-01. doi:10.1111/j.1582-4934.2005.tb00337.x. PMID 15784165.
- ↑ "Identification, classification, and partial characterization of genes in humans and other vertebrates homologous to a fish membrane progestin receptor". Proceedings of the National Academy of Sciences of the United States of America 100 (5): 2237–42. Mar 2003. doi:10.1073/pnas.0436133100. PMID 12601167. Bibcode: 2003PNAS..100.2237Z.
- ↑ "Expression of membrane progesterone receptors (mPR/PAQR) in ovarian cancer cells: implications for progesterone-induced signaling events". Hormones & Cancer 1 (4): 167–76. Aug 2010. doi:10.1007/s12672-010-0023-9. PMID 21761364.
- ↑ "Membrane progesterone receptors (mPRs) mediate progestin induced antimorbidity in breast cancer cells and are expressed in human breast tumors". Hormones & Cancer 3 (3): 101–12. Jun 2012. doi:10.1007/s12672-012-0106-x. PMID 22350867.
- ↑ "Expression profile of heptahelical putative membrane progesterone receptors in epithelial ovarian tumors". Human Pathology 39 (7): 1026–33. Jul 2008. doi:10.1016/j.humpath.2007.11.007. PMID 18479732.
- ↑ "Regulated expression of putative membrane progestin receptor homologues in human endometrium and gestational tissues". The Journal of Endocrinology 187 (1): 89–101. Oct 2005. doi:10.1677/joe.1.06242. PMID 16214944.
- ↑ "Expression and hormonal regulation of membrane progesterone receptors in human astrocytoma cells". The Journal of Steroid Biochemistry and Molecular Biology 154: 176–85. Nov 2015. doi:10.1016/j.jsbmb.2015.08.006. PMID 26275946.
 |