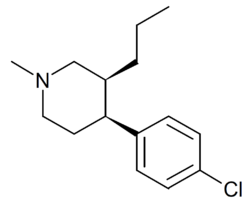
Chemistry:1-Methyl-3-propyl-4-(p-chlorophenyl)piperidine
 | |
| Identifiers | |
|---|---|
| |
| CAS Number |
|
| PubChem CID | |
| ChemSpider | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C15H22ClN |
| Molar mass | 251.80 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
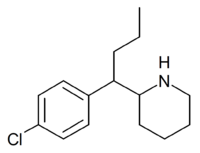
1-Methyl-3-propyl-4-(p-chlorophenyl)piperidine is a drug developed by a team led by Alan Kozikowski, which acts as a potent dopamine reuptake inhibitor, and was developed as a potential therapeutic agent for the treatment of cocaine addiction.[1] As with related compounds such as nocaine, it is a structurally simplified derivative of related phenyltropane compounds.[2] Its activity at the serotonin and noradrenaline transporters has not been published, though most related 4-phenylpiperidine derivatives are relatively selective for inhibiting dopamine reuptake over the other monoamine neurotransmitters. While several of its isomers are active, the (3S,4S)-enantiomer is by far the most potent.[3][4] The rearranged structural isomer 2-[1-(4-chlorophenyl)butyl]piperidine is also a potent inhibitor of dopamine reuptake.[5]
See also
- 4-Fluoropethidine
- Allylprodine
- JZ-IV-10
- N,O-Dimethyl-4-(2-naphthyl)piperidine-3-carboxylate
- WY-46824
References
- ↑ Kozikowski AP, Araldi GL, "Analogs of cocaine", US patent 6180648, issued 2001-01-30
- ↑ "Synthesis and biological properties of new 2beta-alkyl- and 2beta-aryl-3-(substituted phenyl)tropane derivatives: stereochemical effect of C-3 on affinity and selectivity for neuronal dopamine and serotonin transporters". Journal of Medicinal Chemistry 41 (25): 4973–82. December 1998. doi:10.1021/jm9802564. PMID 9836615.
- ↑ "Chemistry and pharmacology of the piperidine-based analogues of cocaine. Identification of potent DAT inhibitors lacking the tropane skeleton". Journal of Medicinal Chemistry 41 (11): 1962–9. May 1998. doi:10.1021/jm980028+. PMID 9599245.
- ↑ Kozikowski AP, Araldi GL, Tamiz AP, "Monomeric and dimeric heterocycles, and therapeutic uses thereof", US patent 6440996, issued 2002-08-27, assigned to Georgetown University
- ↑ "Slow-onset, long-duration, alkyl analogues of methylphenidate with enhanced selectivity for the dopamine transporter". Journal of Medicinal Chemistry 50 (2): 219–32. January 2007. doi:10.1021/jm0608614. PMID 17228864.
 |
