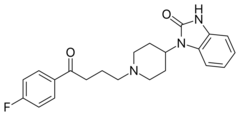
Chemistry:Benperidol
 | |
 | |
| Clinical data | |
|---|---|
| Trade names | Anquil, Frenactil |
| AHFS/Drugs.com | International Drug Names |
| Routes of administration | Oral |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Elimination half-life | 8 hours |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C22H24FN3O2 |
| Molar mass | 381.451 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| | |
Benperidol, sold under the trade name Anquil[1] among others, is a typical antipsychotic primarily used to treat hypersexuality syndromes[2] and can be used to treat schizophrenia.[3] It is a highly potent butyrophenone derivative and is the most potent neuroleptic in the European market, with chlorpromazine equivalency as high as 75 to 100 (about 150 to 200% the potency per dose of haloperidol).[4] It is sometimes prescribed to sex offenders as a condition of their parole, as an alternative to anti-androgen drugs such as cyproterone acetate.[5]
Benperidol was discovered by Janssen Pharmaceutica in 1961 and has been marketed since 1966. It is mainly used in Germany , but it is also available in Belgium, Greece, Italy, the Netherlands, and the United Kingdom .[6]
Pharmacology
Pharmacodynamics
Benperidol is a strong dopamine receptor antagonist (D2 (Ki 0.027 nM) and D4 (Ki 0.066 nM))[7] with weaker serotonin receptor antagonism (5-HT2A (Ki 3.75 nM))[7]. In high doses, it has antihistaminergic and alpha-adrenergic properties. It possesses minimal anticholinergic properties.[8]
| Site | Ki (nM) | Action | Ref |
|---|---|---|---|
| 5-HT2A | 3.75 | Antagonist | [7] |
| D1 | 4,100 | Antagonist | [7] |
| D2 | 0.027 | Antagonist | [7] |
| D4 | 0.06 | Antagonist | [7] |
Pharmacokinetics
Benperidol is absorbed well and undergoes extensive first pass metabolism. One percent of benperidol is excreted in urine. The half-life of benperidol is 8 hours.[8]
Synthesis
4-(2-Keto-1-benzimidazolinyl)piperidine [20662-53-7] (1) is alkylated with 4-Chloro-4'-Fluorobutyrophenone [3874-54-2] (2).
See also
- Timiperone has a similar chemical structure with a thiourea group instead of a urea group.
- Pimozide & Bezitramide (& Oxiperomide & Neflumozide) are also made from 4-(1-Benzimidazolinone)piperidine precursor
- Droperidol is similar, but has a tetrahydropyridine ring.
References
- ↑ "Benperidol - a drug for sexual offenders?". Drug and Therapeutics Bulletin (BMJ Publishing Group Ltd) 12 (3): 12. 1974-02-01. doi:10.1136/dtb.12.3.12. PMID 4457302.
- ↑ British National Formulary (49th), British Medical Association 2005 p 183
- ↑ "[Benperidol and promazine: a "double blind" comparative study in mental geriatrics]" (in fr). Acta Neurologica et Psychiatrica Belgica 63: 839–43. October 1963. PMID 14092279.
- ↑ (in de) Neuroleptika: pharmakologische Grundlagen, klinisches Wissen und therapeutisches Vorgehen; mit 136 Tabellen.. Wiss. Verlag-Ges.. 2001. ISBN 3-8047-1773-X.
- ↑ "Endocrine changes in male sexual deviants after treatment with anti-androgens, oestrogens or tranquillizers". The Journal of Endocrinology 67 (2): 179–88. November 1975. doi:10.1677/joe.0.0670179. PMID 1107462.
- ↑ "NCATS Inxight Drugs — BENPERIDOL". https://drugs.ncats.io/drug/97O6X78C53.
- ↑ 7.0 7.1 7.2 7.3 7.4 7.5 "Dopamine Targeting Drugs for the Treatment of Schizophrenia: Past, Present and Future". Current Topics in Medicinal Chemistry 16 (29): 3385–3403. December 2016. doi:10.2174/1568026616666160608084834. PMID 27291902.
- ↑ 8.0 8.1 "Benperidol for schizophrenia". The Cochrane Database of Systematic Reviews 2005 (2): CD003083. April 2005. doi:10.1002/14651858.CD003083.pub2. PMID 15846648.
- ↑ BE patent 626307 (1963 to Janssen), C.A. 60, 10690c (1964), corresp. to "1-(1-aroylpropyl-4-piperidyl)-2-benzimidazolinones and related compounds" GB patent 989755, published 1965-04-22, assigned to N.V. Research Laboratorium Dr. C. Janssen.
 |


