Biology:Aquaporin-4
 Generic protein structure example |
Aquaporin-4, also known as AQP-4, is a water channel protein encoded by the AQP4 gene in humans.[1] AQP-4 belongs to the aquaporin family of integral membrane proteins that conduct water through the cell membrane. A limited number of aquaporins are found within the central nervous system (CNS): AQP1, 3, 4, 5, 8, 9, and 11, but more exclusive representation of AQP1, 4, and 9 are found in the brain and spinal cord.[2] AQP4 shows the largest presence in the cerebellum and spinal cord grey matter.[3] In the CNS, AQP4 is the most prevalent aquaporin channel, specifically located at the perimicrovessel astrocyte foot processes, glia limitans, and ependyma.[4] In addition, this channel is commonly found facilitating water movement near cerebrospinal fluid and vasculature.[5]
Aquaporin-4 was first identified in 1986. It was the first evidence of the existence of water transport channels.[6] The method that was used to discover the existence of the transport channels was through knockout experiments. With this technique they were able to show the significant role of AQP4 in CNS injuries and brain water imbalances.[2] In 1994 the channel was successfully cloned and initially named Mercury-Insensitive Water Channel.[7]
Structure
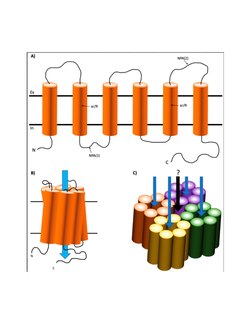
The structure of AQP4 consists of six-transmembrane domains and five connecting loops to form the channel. Through x-ray crystallography, it was found that “each AQP4 monomer consists of six helical, membrane-spanning domains and two short helical segments surrounding a narrow aqueous pore.”[8] At the narrowest point the aqueous pore measures 2.8 angstroms, just large enough for the single-file passage of water molecules. While each monomer is individually capable of water transport, the quaternary structure of the channel is a tetramer.[3] The assembly of AQP4 monomers into tetramers is similar to other aquaporin channels.[9] In addition, AQP4 has two distinct structural isoforms located in the CNS: M1 and M23.[2] Both form homo- and hetero-tetramers that are permeable to water.[2] M23 isoforms are larger square arrays in the endfoot membranes of astrocytes compared to M1 isoforms, which are smaller and more unstable. The aquaporin-4 tetramers accumulate to transform into orthogonal arrays of particle (OAPs) in the cell plasma membrane.[8]
Tissue and cellular distribution
Aquaporin-4 is the most common aquaporin in the brain, spinal cord, and optic nerve.[7] It is highly expressed in the human body primarily at the end-feet of astrocytes.[8] Additionally, AQP4 can also be located in epithelial cells of many organs throughout the human body, such as the kidney, intestine, salivary glands, sensory organs, and skeletal muscles.[6] In these specific cases of epithelial cell expression, AQP4 is concentrated within the basolateral membrane layer of these locations.[9]
Furthermore, AQP4 also plays a role in the supportive cells of sensory organs, such as the retina, inner ear, and olfactory epithelium.[8] Within the retina, AQP4 is highly concentrated where the processes of Müller cells have a basal lamina around blood vessels and inner limiting membrane[6] and to a lesser degree in the inner and outer plexiform layers.[10]
AQP4 is also expressed in astrocytes and is upregulated by direct insult to the central nervous system.[11] Specifically within the central nervous system (CNS), AQP4 can be found along the spinal cord and serves as the main water channel.[2] The AQP4 channels are highly concentrated in the blood-brain barrier (BBB), as well as in other cerebrospinal fluid barriers.[12]
In the kidneys, AQP4 is primarily found in the inner medulla, and shows little to no presence in the outer medulla and cortex.[13] It is constitutively expressed in the basolateral cell membrane of principal collecting duct cells and provide a pathway for water to exit these cells.[14]
Function
Aquaporin-4's overall function is to provide fast water transportation as well as maintain homeostatic balance within the central nervous system. This channel can transport water up to speeds of 3E9 molecules per second.[3] It is the primary water channel protein that reconciles the homeostasis of water in the CNS.[2] AQP4 may be involved in a variety of physiological processes such as waste removal (glymphatic system) and fine-tuning of potassium homeostasis.[12] Water flowing into and out of the brain or spinal cord is assisted by AQP4.[2] Here, AQP4 channels respond passively to osmotic gradients. In addition, they play a role in brain water transport, cell migration, brain edema, metabolism and cell homeostasis.[15]
Other systems are also regulated by AQP4. Within the inner ear, the main role is to provide osmotic balance in supporting epithelium cells within the organ of Corti by recycling K+.[6] Another specific role AQP4 plays is to help odorant molecules bind to target receptors and binding proteins within olfactory epithelium.[6] Within the retina, the role of AQP-4 is to maintain homeostasis.[6] Aquaporin-4 is essential in the formation of memory as well as synaptic plasticity.[12] Other performances that aquaporin-4 is involved in are synaptic plasticity, astrocyte migration, regulation of extracellular space volume, and the homeostasis of potassium.[12]
Clinical significance
The condition known as neuromyelitis optica, NMO, is a rare demyelinating, inflammatory disorder of the CNS that primarily affects the optic nerves and spinal cord of individuals.[16] Aquaporin-4 is the predominant autoimmune target in 2/3 neuromyelitis optica and higher AQP4 autoantibody levels are associated with the occurrence of optic neuritis (ON),[17] however serum AQP4-IgG titer only moderately reflects disease activity, severity, or neurological prognosis.[18] Specific AQP4 IgG autoantibody, or NMO-IgG, binds to the extracellular surface of AQP4.[8] This binding provides an opening for the development of targeted therapeutics in NMO.[8] Therapy options are immunosuppression, such as corticosteroids and azathioprine immunosuppressive drugs, immunomodulation, and plasma exchange.[8] A recent serum antibody (anti-AQP4) has been detected for patients with NMO, which is currently used to diagnose this condition.[4]
Other clinical significant implications of AQP4 in the human body is the role in the regulation of cerebrospinal fluid (CSF) in the ventricles. Within the ventricles of the brain, AQP4 can be utilized in the removal of excess CSF in conditions such as hydrocephaly.[15] The primary treatment for individuals with hydrocephaly is through the implementation of mechanical shunts into the ventricles to drain the excess fluid. With further research into the role of AQP4, it may be possible to modify the human body's system of upregulation of these channels to help in the reabsorption of CSF without the need to use physically invasive treatments.[15]
Research
Based on work in animal models, aquaporin-4 may have a role in several other diseases including Alzheimer's disease, amyotrophic lateral sclerosis, Parkinson's disease, multiple sclerosis, and epilepsy, and appears to have a role in pathological response to traumatic brain injury and stroke.[12]
In rodent models, AQP4 appears plays a role in both the development and resolution of the cerebral edema that occurs following an injury like TBI or stroke and around brain tumors.[4][9] In comparison with wild-type mice, double knockout mice exhibited different diseases course post brain injury.[12] It indicated reduced intracranial pressure, cell death, water accumulation, astrogliosis, and lesion volume.[12] The expression of aquaporin 4 is reliant on the disease stage of TBI.[12] In an acute stage of TBI, the lack of aquaporin 4 causes an decrease of excess water removal while for later stage TBI results in prevention of severe damage and swelling.[12]
In people who suffer from Alzheimer's disease, amyloid plaques sometimes develop in brain arteries—a condition is referred to as cerebral amyloid angiopathy, or CAA. Animal studies have found that the severity of CAA increases or decreases depending on aquaporin-4 expression. When there is an decrease in AQP4, CAA severity increases and vice versa; it is not known what causes changes in AQP4 expression levels, nor whether this is part of the disease process or an effort of the brain to adapt.[12] In animal models of amyotrophic lateral sclerosis, AQP4 is overexpressed in the brainstem, cortex, and gray matter of the spinal cord which results in swollen astrocytes; the reason for this is not understood.[12]
Knockout mice display cognition problems; there is disruption in memory consolidation as well as disruption between memory acquisition, spatial recognition, and memory of where an object was after it has been moved.[12]
References
- ↑ "Molecular characterization of an aquaporin cDNA from brain: candidate osmoreceptor and regulator of water balance". Proceedings of the National Academy of Sciences of the United States of America 91 (26): 13052–6. December 1994. doi:10.1073/pnas.91.26.13052. PMID 7528931. Bibcode: 1994PNAS...9113052J.
- ↑ 2.0 2.1 2.2 2.3 2.4 2.5 2.6 "Aquaporins in the Spinal Cord". International Journal of Molecular Sciences 17 (12): 2050. December 2016. doi:10.3390/ijms17122050. PMID 27941618.
- ↑ 3.0 3.1 3.2 Halsey, Andrea; Conner, Alex; Bill, Roslyn; Logan, Ann; Ahmed, Zubair (2018-10-18). "Aquaporins and Their Regulation after Spinal Cord Injury". Cells 7 (10): 174. doi:10.3390/cells7100174. ISSN 2073-4409. PMID 30340399.
- ↑ 4.0 4.1 4.2 "Aquaporin-4 in brain and spinal cord oedema". Neuroscience 168 (4): 1036–46. July 2010. doi:10.1016/j.neuroscience.2009.08.019. PMID 19682555.
- ↑ Hubbard, Jacqueline A.; Hsu, Mike S.; Seldin, Marcus M.; Binder, Devin K. (2015-10-15). "Expression of the Astrocyte Water Channel Aquaporin-4 in the Mouse Brain". ASN Neuro 7 (5): 175909141560548. doi:10.1177/1759091415605486. ISSN 1759-0914. PMID 26489685.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 "Aquaporin-4 in Astroglial Cells in the CNS and Supporting Cells of Sensory Organs-A Comparative Perspective". International Journal of Molecular Sciences 17 (9): 1411. August 2016. doi:10.3390/ijms17091411. PMID 27571065.
- ↑ 7.0 7.1 Mader, Simone; Brimberg, Lior (2019-01-27). "Aquaporin-4 Water Channel in the Brain and Its Implication for Health and Disease". Cells 8 (2): 90. doi:10.3390/cells8020090. ISSN 2073-4409. PMID 30691235.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 "Biology of AQP4 and anti-AQP4 antibody: therapeutic implications for NMO". Brain Pathology 23 (6): 684–95. November 2013. doi:10.1111/bpa.12085. PMID 24118484.
- ↑ 9.0 9.1 9.2 "Aquaporin-4 and Cerebrovascular Diseases". International Journal of Molecular Sciences 17 (8): 1249. August 2016. doi:10.3390/ijms17081249. PMID 27529222.
- ↑ "Aquaporin-4 water channel protein in the rat retina and optic nerve: polarized expression in Müller cells and fibrous astrocytes". The Journal of Neuroscience 18 (7): 2506–19. April 1998. doi:10.1523/JNEUROSCI.18-07-02506.1998. PMID 9502811.
- ↑ "Aquaporin-4 in the central nervous system: cellular and subcellular distribution and coexpression with KIR4.1". Neuroscience 129 (4): 905–13. 2004. doi:10.1016/j.neuroscience.2004.08.053. PMID 15561407.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 "The role of aquaporin-4 in synaptic plasticity, memory and disease". Brain Research Bulletin 136: 118–129. January 2018. doi:10.1016/j.brainresbull.2017.02.011. PMID 28274814.
- ↑ Terris, J.; Ecelbarger, C. A.; Marples, D.; Knepper, M. A.; Nielsen, S. (1995-12-01). "Distribution of aquaporin-4 water channel expression within rat kidney". American Journal of Physiology. Renal Physiology 269 (6): F775–F785. doi:10.1152/ajprenal.1995.269.6.f775. ISSN 1931-857X. PMID 8594871.
- ↑ "The aquaporin family of water channels in kidney". Nephrologie 17 (7): 409–15. 1996. PMID 8987045.
- ↑ 15.0 15.1 15.2 "Hydrocephalus: the role of cerebral aquaporin-4 channels and computational modeling considerations of cerebrospinal fluid". Neurosurgical Focus 41 (3): E8. September 2016. doi:10.3171/2016.7.FOCUS16191. PMID 27581320.
- ↑ "Aquaporin-4 antibodies (NMO-IgG) as a serological marker of neuromyelitis optica: a critical review of the literature". Brain Pathology 23 (6): 661–83. November 2013. doi:10.1111/bpa.12084. PMID 24118483.
- ↑ "Quantitative assays for anti-aquaporin-4 antibody with subclass analysis in neuromyelitis optica". Multiple Sclerosis 18 (11): 1541–51. November 2012. doi:10.1177/1352458512443917. PMID 22526930.
- ↑ "Clinical relevance of serum aquaporin-4 antibody levels in neuromyelitis optica". Neurochemical Research 38 (5): 997–1001. May 2013. doi:10.1007/s11064-013-1009-0. PMID 23456674.
Further reading
- "Roles of aquaporin-4 isoforms and amino acids in square array assembly". Biochemistry 48 (25): 5785–93. June 2009. doi:10.1021/bi802231q. PMID 19445480.
- "Time course of upregulation of inflammatory mediators in the hemorrhagic brain in rats: correlation with brain edema". Neurochemistry International 57 (3): 248–53. October 2010. doi:10.1016/j.neuint.2010.06.002. PMID 20541575.
- "A role for aquaporin-4 in fluid regulation in the inner retina". Visual Neuroscience 26 (2): 159–65. 2009. doi:10.1017/S0952523809090038. PMID 19366470.
- "Association of the HLA-DPB1*0501 allele with anti-aquaporin-4 antibody positivity in Japanese patients with idiopathic central nervous system demyelinating disorders". Tissue Antigens 73 (2): 171–6. February 2009. doi:10.1111/j.1399-0039.2008.01172.x. PMID 19140826.
- "Investigating the genetic role of aquaporin4 gene in migraine". The Journal of Headache and Pain 10 (2): 111–4. April 2009. doi:10.1007/s10194-009-0100-z. PMID 19209385.
- "Aquaporin-4, homeostasis, and neurologic disease". Neurology 69 (24): 2266–8. December 2007. doi:10.1212/01.wnl.0000286385.59836.e2. PMID 18071147.
- "Crystal structure of human aquaporin 4 at 1.8 A and its mechanism of conductance". Proceedings of the National Academy of Sciences of the United States of America 106 (18): 7437–42. May 2009. doi:10.1073/pnas.0902725106. PMID 19383790. Bibcode: 2009PNAS..106.7437H.
- "Aquaporin-4 expression is severely reduced in human sarcoglycanopathies and dysferlinopathies". Cell Cycle 7 (14): 2199–207. July 2008. doi:10.4161/cc.7.14.6272. PMID 18641458.
- "Changes in ocular aquaporin-4 (AQP4) expression following retinal injury". Molecular Vision 14: 1770–83. September 2008. PMID 18836575.
- "Novel variants in human Aquaporin-4 reduce cellular water permeability". Human Molecular Genetics 17 (15): 2379–89. August 2008. doi:10.1093/hmg/ddn138. PMID 18511455.
- "Aquaporin-4 expression is increased in edematous meningiomas". Journal of Clinical Neuroscience 16 (3): 441–3. March 2009. doi:10.1016/j.jocn.2008.04.028. PMID 19153045.
- "A case of NMO seropositive for aquaporin-4 antibody more than 10 years before onset". Neurology 72 (22): 1960–1. June 2009. doi:10.1212/WNL.0b013e3181a82621. PMID 19487655.
- "[Neuromyelitis optica and anti-aquaporin 4 antibody--an overview]" (in ja). Brain and Nerve = Shinkei Kenkyu No Shinpo 60 (5): 527–37. May 2008. PMID 18516975.
- "Aquaporins (water channels): role in vasopressin-activated water transport". Proceedings of the Society for Experimental Biology and Medicine 219 (3): 183–99. December 1998. doi:10.3181/00379727-219-44332. PMID 9824541.
- "Hypercomplementemia at relapse in patients with anti-aquaporin-4 antibody". Multiple Sclerosis 15 (3): 304–10. March 2009. doi:10.1177/1352458508099139. PMID 19028829.
- "Aquaporin-4 autoantibodies in a paraneoplastic context". Archives of Neurology 65 (5): 629–32. May 2008. doi:10.1001/archneur.65.5.629. PMID 18474738.
- "Aquaporin-4 autoimmune syndrome and anti-aquaporin-4 antibody-negative opticospinal multiple sclerosis in Japanese". Multiple Sclerosis 15 (7): 834–47. July 2009. doi:10.1177/1352458509104595. PMID 19465451.
- "Neuromyelitis optica pathogenesis and aquaporin 4". Journal of Neuroinflammation 5: 22. May 2008. doi:10.1186/1742-2094-5-22. PMID 18510734.
- "Narcolepsy as an initial manifestation of neuromyelitis optica with anti-aquaporin-4 antibody". Journal of Neurology 256 (2): 287–8. February 2009. doi:10.1007/s00415-009-0139-4. PMID 19266146.
- "Differential expression of aquaporin-4 in human gastric normal and cancer tissues". Gastroenterologie Clinique et Biologique 33 (1 Pt 1): 72–6. January 2009. doi:10.1016/j.gcb.2008.07.010. PMID 19112001.
- "Phosphorylation in the C-terminal domain of Aquaporin-4 is required for Golgi transition in primary cultured astrocytes". Biochemical and Biophysical Research Communications 377 (2): 463–468. December 2008. doi:10.1016/j.bbrc.2008.09.155. PMID 18854171.
External links
- Aquaporin+4 at the US National Library of Medicine Medical Subject Headings (MeSH)
- Human AQP4 genome location and AQP4 gene details page in the UCSC Genome Browser.
- Overview of all the structural information available in the PDB for UniProt: P55087 (Aquaporin-4) at the PDBe-KB.
 |