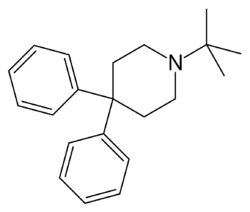
Chemistry:Budipine
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEMBL | |
| Chemical and physical data | |
| Formula | C21H27N |
| Molar mass | 293.454 g·mol−1 |
| 3D model (JSmol) | |
| |
| |
| (verify) | |
Budipine (brand name Parkinsan) is an antiparkinson agent marketed for the treatment of Parkinson's disease.[2][3][4]
While its exact mechanism of action is not well characterized,[2] it is believed to be an NMDA receptor antagonist,[5][6] but also promoting the synthesis of dopamine.[7]
Because it provides additional benefits relative to existing treatments, it probably does not precisely mimic the mechanism of an existing known treatment.[7][8]
Synthesis
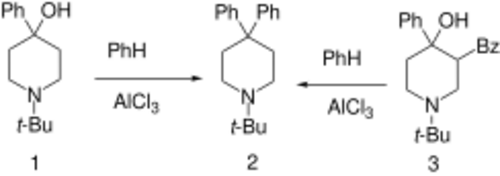
Budipine can be prepared from the 1-tert-butyl-4-piperidone [1465-76-5] directly by treatment with benzene in the presence triflic acid.[9] This method of synthesis enables a 99% yield of product.
4-Phenyl-1-t-butyl-4-piperidinol,[11] (1)
1-t-butyl-3-benzoyl-4-phenyl-4-piperidinol [81831-81-4] (3)
See also
- AD-1211
- Delucemine
- Diphenidine
- Ephenidine
- Fluorolintane
- Lanicemine
- Methoxphenidine (MXP)
- MT-45
- Remacemide
References
- ↑ Martindale: The Complete Drug Reference. (35th ed.). London: Pharmaceutical Press. 2007. ISBN 978-0-85369-687-2.
- ↑ 2.0 2.1 "Budipine in Parkinson's tremor". Journal of the Neurological Sciences 248 (1–2): 53–55. October 2006. doi:10.1016/j.jns.2006.05.039. PMID 16784759.
- ↑ "Clinical efficacy of budipine in Parkinson's disease". Diagnosis and Treatment of Parkinson's Disease — State of the Art. Journal of Neural Transmission. Supplementa. 56. 1999. 75–82. doi:10.1007/978-3-7091-6360-3_3. ISBN 978-3-211-83275-2.
- ↑ "Budipine". AdisInsight. Springer Nature Switzerland AG. http://adisinsight.springer.com/drugs/800003459.
- ↑ "The antiparkinsonian drug budipine binds to NMDA and sigma receptors in postmortem human brain tissue". Journal of Neural Transmission. Supplementum 46: 131–137. 1995. PMID 8821048.
- ↑ "Neuroprotection by NMDA receptor antagonists in a variety of neuropathologies". Current Drug Targets 2 (3): 241–271. September 2001. doi:10.2174/1389450013348335. PMID 11554551.
- ↑ 7.0 7.1 "Budipine provides additional benefit in patients with Parkinson disease receiving a stable optimum dopaminergic drug regimen". Archives of Neurology 59 (5): 803–806. May 2002. doi:10.1001/archneur.59.5.803. PMID 12020263.
- ↑ "Effects of amantadine and budipine on antidepressant drug-evoked changes in extracellular dopamine in the frontal cortex of freely moving rats". Brain Research 1117 (1): 206–212. October 2006. doi:10.1016/j.brainres.2006.07.039. PMID 16996043.
- ↑ Klumpp, D. A., Garza, M., Jones, A., Mendoza, S. (1 September 1999). "Synthesis of Aryl-Substituted Piperidines by Superacid Activation of Piperidones". The Journal of Organic Chemistry. 64 (18): 6702–6705. doi:10.1021/jo990454i.
- ↑ "[Synthesis, physical-chemical properties and pharmacologically-oriented screening studies on budipine and related 4,4-diphenylpiperidines]" (in German). Arzneimittel-Forschung 34 (3): 233–240. 1984. PMID 6539602.
- ↑ "4-Phenyl-1-t-butyl-4-piperidinol". PubChem. U.S. National Library of Medicine. https://pubchem.ncbi.nlm.nih.gov/compound/20536606.
 |