Chemistry:Trihexyphenidyl
Trihexyphenidyl (THP, benzhexol, trihex, marketed as Artane and others) is an antispasmodic drug used to treat stiffness, tremors, spasms, and poor muscle control. It is an agent of the antimuscarinic class and is often used in management of Parkinson's disease. It was approved by the FDA for the treatment of Parkinson's in the US in 1949.[1][2]
Trihexyphenidyl is a therapeutic alternative on the World Health Organization's List of Essential Medicines.[3]
Medical uses
Trihexyphenidyl is used for the symptomatic treatment of Parkinson's disease in mono and combination therapy.[4]
Trihexyphenidyl has also been prescribed for essential tremor and akathisia.[5][6]
In pediatrics, it has been used for children with dystonia due to cerebral palsy,[7][8][9] and to control drooling.[10]
In organophosphate poisoning, trihexyphenidyl is a more effective antidote than atropine to counteract the cholinergic crisis, seizures, and neuropathology.[11]
Contraindications
Contraindications include according to the Therapeutic Goods Administration Australia from 2022:[12]
- Hypersensitivity to trihexyphenidyl
- Narrow angle glaucoma
- Ileus (disruption of the normal propulsive ability of the intestine)
- Caution: People with obstructive diseases of the urogenital tract, people with a known history of seizures and those with potentially dangerous tachycardia
Adverse effects
Dose-dependent side effects are frequent, but typically lessen over time as the body adapts to the medication. All of the following symptoms considered, Artane has been shown to dramatically and consistently improve neurologic defects in people aged 16–86 over the course of five years.[13] People who are older or who have psychiatric conditions may become confused or develop delirium. Side effects include but are not limited to:[14]
- Central nervous system: drowsiness, vertigo, headache, and dizziness are frequent. With high doses nervousness, agitation, anxiety, delirium, and confusion are noted. Trihexyphenidyl may be abused due to a short acting mood-elevating and euphoric effect. The normal sleep architecture may be altered (REM sleep depression). Trihexyphenidyl may lower the seizure-threshold.
- Peripheral side effects: dry mouth, impaired sweating, abdominal discomfort, nausea, and constipation are frequent. Tachycardia or heart palpitations (fast heart rate) may be noted. Allergic reactions are rare, but may occur. Many of these peripheral symptoms, especially considering an acute increase in anxiety with various physical complaints, as well as evidence of orthostatic hypotension and tachycardia are indicative of withdrawal, especially in people with psychiatric conditions [15]
- Eyes: trihexyphenidyl causes mydriasis with or without photophobia. It may precipitate narrow angle glaucoma or cause blurred vision.
- Tolerance may develop during therapy which requires dose adjustments.
- Striated musculature and weight gain.
Overdose
This section needs more medical references for verification or relies too heavily on primary sources. (May 2017) |
Trihexyphenidyl and other antiparkinsonian drugs are known to be substances of abuse. This is true both in abusers of other substances and in chronic schizophrenics, the latter being infrequent abusers of other drugs.[16]
Overdose mimics an atropine intoxication with dryness of mucous membranes, red face, bowel and bladder paralysis, and hyperthermia in high doses. Central nervous system consequences are agitation, confusion, and hallucinations. An untreated overdose may be fatal, particularly in children. Premortal signs are respiratory depression, arrhythmia and cardiac arrest.
A case report of 24-hour long arrhythmia was treated with verapamil.[17]
Excessive myoclonus can be complicated by rhabdomyolysis; in one case risk was increased due to concomitant use of risperidone.[18]
Interactions
This section needs more medical references for verification or relies too heavily on primary sources. (May 2017) |
- Other anticholinergic drugs (e.g. spasmolytics, antihistamines, TCAs) : Side effects of trihexyphenidyl may be increased.
- Quinidine : Increased anticholinergic action (particular on AV conduction).
- Antipsychotics : Long term use of trihexyphenidyl may mask or increase the risk of tardive dyskinesia.
- Pethidine (meperidine) : Central effects and side effects of pethidine may be increased.
- Metoclopramide : Action of metoclopramide is decreased.
- Alcohol : Risk of serious intoxication.
Pharmacology
Pharmacodynamics
Trihexyphenidyl is an anticholinergic.[19] It is specifically an antimuscarinic and acts as a non-selective antagonist of all five muscarinic acetylcholine receptors.[19] However, its antagonistic activity is much stronger at the muscarinic acetylcholine M1 and M4 receptors, and it can be described as selective for these receptors.[19][20]
The exact mechanism of action in parkinsonian syndromes is not precisely understood, but it is known that trihexyphenidyl blocks efferent impulses in parasympathetically innervated structures like smooth muscles (spasmolytic activity), salivary glands, and eyes (mydriasis). In higher doses direct central inhibition of cerebral motor centers may contribute. In very high doses central toxicity as seen in atropine overdose is noted.
It possibly also binds to dopamine receptors.[21]
Pharmacokinetics
Trihexyphenidyl is rapidly absorbed from the gastrointestinal tract. The onset of action is within 1 hour after oral dosing. The peak activity is noted after 2 to 3 hours.[22] The duration of action of one single dose is 6 to 12 hours in a dose dependent manner. It is excreted in the urine, probably as unchanged drug. More precise data in animals and humans have so far not been determined.[23][24]
History
Trihexyphenidyl has been clinically relevant in trials pertaining to Parkinson's disease since 1949.[25]
In the US, the Food and Drug Association approved Artane, or its generic form Trihexyphenidyl HCL, in June 2003, for the clinical use of all types of parkinsonism.[26]
Society and culture
Recreational use
The neurologist Oliver Sacks reported using the drug recreationally in the 1960s.[27] He recalled taking "a large dose" knowing full well the drug was intended for people with Parkinson's. More recounts of Dr. Sacks' experiences — including experimentation with mescaline, psilocybin, LSD, and probably DMT[28] — have been compared in his book Hallucinations.
During the 1970s, trihexyphenidyl (trade name Parkan) was the most popular recreationally used prescription drug in Hungary.[29]
In a 2008 news report, trihexyphenidyl was seen to be used for recreational purposes among Iraqi soldiers and police, among other prescription drugs. The report states that the drugs were taken to relieve combat stress reaction.[30] Although that may be the case for some, others used Artane as a substitute or more intense version of LSD. This was especially prevalent in the 1960s, according to a report in "The New Yorker". Similarly to those in Iraqi forces, some of the appeal was that the individual may retain partial control while under the influence.[31] It is still diverted from its primary use, in combination with other drugs, on the Réunion island (France).[32]
Chemistry
Trihexyphenidyl can be synthesized in two ways, one linear and one convergent synthesis.
In the first way, the initial 2-(1-piperidino)propiophenone is synthesized in turn by the aminomethylation of acetophenone using paraformaldehyde and piperidine in a Mannich reaction. In the second step the 2-(1-piperidino)propiophenone is reacted with cyclohexylmagnesium bromide in a Grignard reaction.[33]
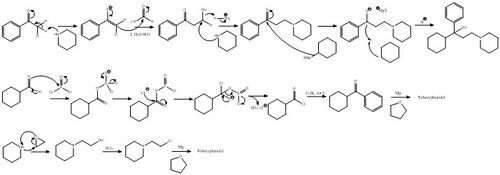
Stereochemistry
Trihexyphenidyl has a chiral center and two enantiomers. Medications are racemates.[34]
| Enantiomers | |
|---|---|
 CAS number: 40520-25-0 |
 CAS number: 40520-24-9 |
References
- ↑ "TGA eBS - Product and Consumer Medicine Information Licence". https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2016-PI-01605-1&d=202008201016933.
- ↑ "New Drug Application Approval Notice". U.S. Food and Drug Administration. 2003-05-25. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/006773_S036_ARTANE.pdf.
- ↑ The selection and use of essential medicines, 2025: WHO Model List of Essential Medicines, 24th list. Geneva: World Health Organization. 2025.
- ↑ "Movement disorders". The Medical Clinics of North America. Common Neurologic Disorders 93 (2): 371–88, viii. March 2009. doi:10.1016/j.mcna.2008.09.002. PMID 19272514.
- ↑ "[Drug-Induced Akathisia]". Brain and Nerve = Shinkei Kenkyu No Shinpo 69 (12): 1417–1424. December 2017. doi:10.11477/mf.1416200927. PMID 29282345.
- ↑ "Drug-induced movement disorders". Australian Prescriber 42 (2): 56–61. April 2019. doi:10.18773/austprescr.2019.014. PMID 31048939.
- ↑ "Trihexyphenidyl improves motor function in children with dystonic cerebral palsy: a retrospective analysis". Journal of Child Neurology 26 (7): 810–816. July 2011. doi:10.1177/0883073810392582. PMID 21498790.
- ↑ "Prospective open-label clinical trial of trihexyphenidyl in children with secondary dystonia due to cerebral palsy". Journal of Child Neurology 22 (5): 530–7. May 2007. doi:10.1177/0883073807302601. PMID 17690057.
- ↑ ""Complex I Deficiency". Mitochondrial Case Studies: Underlying Mechanisms and Diagnosis.. Academic Press. November 2015. pp. 257–64. ISBN 978-0-12-801149-2.
- ↑ "Anticholinergic medications for reducing drooling in children with developmental disability". Developmental Medicine and Child Neurology 62 (3): 346–353. March 2020. doi:10.1111/dmcn.14350. PMID 31495925.
- ↑ "(R,S)-trihexyphenidyl, acting via a muscarinic receptor-independent mechanism, inhibits hippocampal glutamatergic and GABAergic synaptic transmissions: Potential relevance for treatment of organophosphorus intoxication". Neuropharmacology 239. November 2023. doi:10.1016/j.neuropharm.2023.109684. PMID 37549771.
- ↑ "TGA eBS - Product and Consumer Medicine Information Licence". Therapeutic Goods Administration. Australian Government. July 2022. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2016-PI-01605-1&d=202008201016933.
- ↑ "Five year follow-up of treatment with trihexyphenidyl (artane); outcome in four hundred eleven cases of paralysis agitans". Journal of the American Medical Association 154 (16): 1334–6. April 1954. doi:10.1001/jama.1954.02940500014005. PMID 13151847.
- ↑ "Trihexyphenidyl". First Databank Inc. nd. http://www.webmd.com/drugs/2/drug-8720/trihexyphenidyl-oral/details#side-effects.
- ↑ "Trihexyphenidyl". Toxnet. http://toxnet.nlm.nih.gov/cgi-bin/sis/search/r?dbs+hsdb:@term+@rn+@rel+144-11-6.
- ↑ "Misuse of Anticholinergic Medications: A Systematic Review". Biomedicines 10 (2): 355. February 2022. doi:10.3390/biomedicines10020355. PMID 35203563.
- ↑ "Anticholinergic overdose induced torsade de pointes successfully treated with verapamil". Japanese Heart Journal 37 (6): 925–931. November 1996. doi:10.1536/ihj.37.925. PMID 9057687.
- ↑ "Multidrug overdose-induced myoclonus complicated by rhabdomyolysis: possible role and mechanism of muscle toxicity of risperidone". Journal of Clinical Pharmacy and Therapeutics 39 (6): 698–700. December 2014. doi:10.1111/jcpt.12205. PMID 25203795.
- ↑ 19.0 19.1 19.2 "DARK Classics in Chemical Neuroscience: Atropine, Scopolamine, and Other Anticholinergic Deliriant Hallucinogens". ACS Chem Neurosci 10 (5): 2144–2159. May 2019. doi:10.1021/acschemneuro.8b00615. PMID 30566832.
- ↑ "Binding and functional profiles of the selective M1 muscarinic receptor antagonists trihexyphenidyl and dicyclomine". Br J Pharmacol 89 (1): 83–90. September 1986. doi:10.1111/j.1476-5381.1986.tb11123.x. PMID 2432979.
- ↑ "Addiction, dopamine, and the molecular mechanisms of memory". Neuron 25 (3): 515–32. March 2000. doi:10.1016/S0896-6273(00)81056-9. PMID 10774721.
- ↑ "Trihexyphenidyl Hydrochloride". https://www.drugs.com/monograph/trihexyphenidyl-hydrochloride.html.
- ↑ Watson Laboratories Inc. trihexyphenidyl hydrochloride tablets, USP. prescribing information. Corona, CA; 2005 May.
- ↑ "Trihexyphenidyl". AHFS drug information. Bethesda, MD: American Society of Health-System Pharmacists. 2006. pp. 1256.
- ↑ "Artane therapy for parkinsonism; a preliminary study of results in 117 cases". Journal of the American Medical Association 140 (17): 1317–22. August 1949. doi:10.1001/jama.1949.02900520003002. PMID 18137284.
- ↑ "Approval Package for Application No. 6-773/36". U.S. Food and Drug Association. https://www.accessdata.fda.gov/drugsatfda_docs/nda/2003/006773_S036_ARTANE.pdf.
- ↑ "Oliver Sacks shares his hallucinations", The Guardian (Guardian), 2012-10-30, https://www.theguardian.com/science/blog/2012/oct/30/oliver-sacks-shares-hallucinations
- ↑ "Chapter 6". Hallucinations. Random House Inc.. 2012.
- ↑ "Kábítószerek a szocializmusban". 19 November 2011. https://mult-kor.hu/20111119_kabitoszerfogyasztas_a_szocialista_idoszakban.
- ↑ "Abuse of Prescription Drugs Rises Among Stressed Iraqi Soldiers". The New York Times. 2008-12-20. https://www.nytimes.com/2008/12/21/world/middleeast/21drugs.html?pagewanted=all.
- ↑ "Altered States". The New Yorker (Condé Nast). 20 August 2012. http://www.newyorker.com/magazine/2012/08/27/altered-states-3. Retrieved 7 May 2017.
- ↑ "Psychoactive cocktail consumption on Reunion Island: A case report". Journal of Analytical Toxicology 49 (5): 369–373. February 2025. doi:10.1093/jat/bkaf009. PMID 39953779.
- ↑ "Synthesis of Certain 3-Hydroxy-3-phenylpropylsulfonium Salts. Sulfonium Analogs of Artane (Trihexyphenidyl) and Pathilon (Tridihexethyl Iodide).". Journal of the American Chemical Society 79 (17): 4771–6. September 1957. doi:10.1021/ja01574a048. Bibcode: 1957JAChS..79.4771W.
- ↑ Rote Liste Service GmbH (Hrsg.) (2017). Rote Liste 2017 – Arzneimittelverzeichnis für Deutschland (einschließlich EU-Zulassungen und bestimmter Medizinprodukte). 57. Frankfurt/Main: Rote Liste Service GmbH. pp. 224. ISBN 978-3-946057-10-9.
![]() This article incorporates public domain material from the United States Department of Health and Human Services document "Toxnet:Trihexyphenidyl".
This article incorporates public domain material from the United States Department of Health and Human Services document "Toxnet:Trihexyphenidyl".
 |
