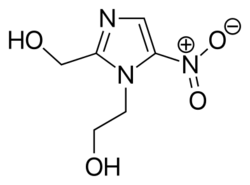
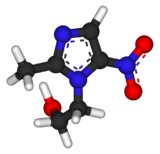
Chemistry:Metronidazole
 | |
 | |
| Clinical data | |
|---|---|
| AHFS/Drugs.com | Monograph |
| MedlinePlus | a689011 |
| License data |
|
| Pregnancy category |
|
| Routes of administration | By mouth, topical, rectal, intravenous, vaginal |
| ATC code | |
| Legal status | |
| Legal status | |
| Pharmacokinetic data | |
| Protein binding | 20%[5][6] |
| Metabolism | Liver[5][6] |
| Metabolites | Hydroxymetronidazole |
| Elimination half-life | 8 hours[5][6] |
| Excretion | Urine (77%), faeces (14%)[5][6] |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| ChemSpider | |
| UNII | |
| KEGG | |
| ChEBI | |
| ChEMBL | |
| NIAID ChemDB | |
| PDB ligand | |
| Chemical and physical data | |
| Formula | C6H9N3O3 |
| Molar mass | 171.156 g·mol−1 |
| 3D model (JSmol) | |
| Melting point | 159 to 163 °C (318 to 325 °F) |
| |
| |
| (verify) | |
Metronidazole, sold under the brand name Flagyl among others, is an antibiotic and antiprotozoal medication.[7] It is used either alone or with other antibiotics to treat pelvic inflammatory disease, endocarditis, and bacterial vaginosis.[7] It is effective for dracunculiasis, giardiasis, trichomoniasis, and amebiasis.[7] It is an option for a first episode of mild-to-moderate Clostridioides difficile colitis if vancomycin or fidaxomicin is unavailable.[7][8] Metronidazole is available orally (by mouth), as a cream or gel, and by slow intravenous infusion (injection into a vein).[7][2]
Common side effects include nausea, a metallic taste, loss of appetite, and headaches.[7] Occasionally seizures or allergies to the medication may occur.[7] Some state that metronidazole should not be used in early pregnancy, while others state doses for trichomoniasis are safe.[1][weasel words] Metronidazole is generally considered compatible with breastfeeding.[1][9]
Metronidazole began to be commercially used in 1960 in France.[10] It is on the World Health Organization's List of Essential Medicines.[11] It is available in most areas of the world.[12] In 2020, it was the 222nd most commonly prescribed medication in the United States, with more than 2 million prescriptions.[13][14]
Medical uses
Metronidazole has activity against some protozoans and most anaerobic bacteria (both Gram-negative and Gram-positive classes) but not the aerobic bacteria.[15][16]
Metronidazole is primarily used to treat: bacterial vaginosis, pelvic inflammatory disease (along with other antibacterials like ceftriaxone), pseudomembranous colitis, aspiration pneumonia, rosacea (topical), fungating wounds (topical), intra-abdominal infections, lung abscess, periodontal disease, amoebiasis, oral infections, giardiasis, trichomoniasis, and infections caused by susceptible anaerobic organisms such as Bacteroides, Fusobacterium, Clostridium, Peptostreptococcus, and Prevotella species.[17] It is also often used to eradicate Helicobacter pylori along with other drugs and to prevent infection in people recovering from surgery.[17]
Metronidazole is bitter and so the liquid suspension contains metronidazole benzoate. This may require hydrolysis in the gastrointestinal tract and some sources speculate that it may be unsuitable in people with diarrhea or feeding-tubes in the duodenum or jejunum.[18][19]
Bacterial vaginosis
Drugs of choice for the treatment of bacterial vaginosis include metronidazole and clindamycin.[20]
Trichomoniasis
The 5-nitroimidazole drugs (metronidazole and tinidazole) are the mainstay of treatment for infection with Trichomonas vaginalis. Treatment for both the infected patient and the patient's sexual partner is recommended, even if asymptomatic. Therapy other than 5-nitroimidazole drugs is also an option, but cure rates are much lower.[21]
Giardiasis
Oral metronidazole is a treatment option for giardiasis, however, the increasing incidence of nitroimidazole resistance is leading to the increased use of other compound classes.[22]
Dracunculus
In the case of Dracunculus medinensis (Guinea worm), metronidazole just eases worm extraction rather than killing the worm.[7]
C. difficile colitis
Initial antibiotic therapy for less-severe Clostridioides difficile infection colitis (pseudomembranous colitis) consists of metronidazole, vancomycin, or fidaxomicin by mouth.[8] In 2017, the IDSA generally recommended vancomycin and fidaxomicin over metronidazole.[8] Vancomycin by mouth has been shown to be more effective in treating people with severe C. difficile colitis.[23]
E. histolytica
Entamoeba histolytica invasive amebiasis is treated with metronidazole for eradication, in combination with diloxanide to prevent recurrence.[24] Although it is generally a standard treatment it is associated with some side effects.[25]
Preterm births
Metronidazole has also been used in women to prevent preterm birth associated with bacterial vaginosis, amongst other risk factors including the presence of cervicovaginal fetal fibronectin (fFN). Metronidazole was ineffective in preventing preterm delivery in high-risk pregnant women (selected by history and a positive fFN test) and, conversely, the incidence of preterm delivery was found to be higher in women treated with metronidazole.[26]
Hypoxic radiosensitizer
In addition to its anti-biotic properties, attempts were also made to use a possible radiation-sensitizing effect of metronidazole in the context of radiation therapy against hypoxic tumors.[27] However, the neurotoxic side effects occurring at the required dosages have prevented the widespread use of metronidazole as an adjuvant agent in radiation therapy.[28] However, other nitroimidazoles derived from metronidazole such as nimorazole with reduced electron affinity showed less serious neuronal side effects and have found their way into radio-onological practice for head and neck tumors in some countries.[29]
Perioral dermatitis
Canadian Family Physician has recommended topical metronidazole as a third-line treatment for the perioral dermatitis either along with or without oral tetracycline or oral erythromycin as first and second line treatment respectively.[30]
Adverse effects
Common adverse drug reactions (≥1% of those treated with the drug) associated with systemic metronidazole therapy include: nausea, diarrhea, weight loss, abdominal pain, vomiting, headache, dizziness, and metallic taste in the mouth. Intravenous administration is commonly associated with thrombophlebitis. Infrequent adverse effects include: hypersensitivity reactions (rash, itch, flushing, fever), headache, dizziness, vomiting, glossitis, stomatitis, dark urine, and paraesthesia.[17] High doses and long-term systemic treatment with metronidazole are associated with the development of leucopenia, neutropenia, increased risk of peripheral neuropathy, and central nervous system toxicity.[17] Common adverse drug reaction associated with topical metronidazole therapy include local redness, dryness and skin irritation; and eye watering (if applied near eyes).[17][31] Metronidazole has been associated with cancer in animal studies.[32][failed verification] In rare cases, it can also cause temporary hearing loss that reverses after cessation of the treatment.[33][34]
Some evidence from studies in rats indicates the possibility it may contribute to serotonin syndrome, although no case reports documenting this have been published to date.[35][36]
Mutagenesis and carcinogenesis
In 2016 metronidazole was listed by the U.S. National Toxicology Program (NTP) as reasonably anticipated to be a human carcinogen.[37] Although some of the testing methods have been questioned, oral exposure has been shown to cause cancer in experimental animals and has also demonstrated some mutagenic effects in bacterial cultures.[37][38] The relationship between exposure to metronidazole and human cancer is unclear.[37][39] One study [40] found an excess in lung cancer among women (even after adjusting for smoking), while other studies [41][42][43] found either no increased risk, or a statistically insignificant risk.[37][44] Metronidazole is listed as a possible carcinogen according to the World Health Organization (WHO) International Agency for Research on Cancer (IARC).[45] A study in those with Crohn's disease also found chromosomal abnormalities in circulating lymphocytes in people treated with metronidazole.[38]
Stevens–Johnson syndrome
Metronidazole alone rarely causes Stevens–Johnson syndrome, but is reported to occur at high rates when combined with mebendazole.[46]
Drug interactions
Alcohol
Consuming alcohol while taking metronidazole has been suspected in case reports to cause a disulfiram-like reaction with effects that can include nausea, vomiting, flushing of the skin, tachycardia, and shortness of breath.[47] People are often advised not to drink alcohol during systemic metronidazole therapy and for at least 48 hours after completion of treatment.[17] However, some studies call into question the mechanism of the interaction of alcohol and metronidazole,[48][49][50] and a possible central toxic serotonin reaction for the alcohol intolerance is suggested.[35] Metronidazole is also generally thought to inhibit the liver metabolism of propylene glycol (found in some foods, medicines, and in many electronic cigarette e-liquids), thus propylene glycol may potentially have similar interaction effects with metronidazole.
Other drug interactions
Metronidazole is a moderate CYP2C9 inhibitor. CYP2C9 is an enzyme of cytochrome P450 family. Therefore, metronidazole may interact with medications metabolized by this enzyme.[51][52][53] Examples of such medications are lomitapide, warfarin, etc.[5]
Pharmacology
Mechanism of action
Metronidazole is of the nitroimidazole class. It inhibits nucleic acid synthesis by forming nitroso radicals, which disrupt the DNA of microbial cells.[5][54] This function only occurs when metronidazole is partially reduced, and because this reduction usually happens only in anaerobic bacteria and protozoans, it has relatively little effect upon human cells or aerobic bacteria.[55]
Pharmacokinetics
Oral metronidazole is approximately 80% bioavailable via the gut and peak blood plasma concentrations occur after one to two hours. Food may slow down absorption but does not diminish it. Of the circulating substance, about 20% is bound to plasma proteins. It penetrates well into tissues, the cerebrospinal fluid, the amniotic fluid and breast milk, as well as into abscess cavities.[54]
About 60% of the metronidazole is metabolized by oxidation to the main metabolite hydroxymetronidazole and a carboxylic acid derivative, and by glucuronidation. The metabolites show antibiotic and antiprotozoal activity in vitro.[54] Metronidazole and its metabolites are mainly excreted via the kidneys (77%) and to a lesser extent via the faeces (14%).[5][6] The biological half-life of metronidazole in healthy adults is eight hours, in infants during the first two months of their lives about 23 hours, and in premature babies up to 100 hours.[54]
The biological activity of hydroxymetronidazole is 30% to 65%, and the elimination half-life is longer than that of the parent compound.[56] The serum half-life of hydroxymetronidazole after suppository was 10 hours, 19 hours after intravenous infusion, and 11 hours after a tablet.[57]
Bacterial resistance
Bacteria may have developed unexpectedly higher resistance to metronidazole.[58][59][60][clarification needed]
History
The drug was initially developed by Rhône-Poulenc in the 1950s[61] and licensed to G.D. Searle.[62] Searle was acquired by Pfizer in 2003.[63] The original patent expired in 1982, but evergreening reformulation occurred thereafter.[64]
Brand name
In India, it is sold under the brand name Metrogyl and Flagyl.[65] In Bangladesh, it is available as Amodis, Amotrex, Dirozyl, Filmet, Flagyl, Flamyd, Metra, Metrodol, Metryl, etc.[66] In Pakistan, it is sold under the brand name of Flagyl and Metrozine.[citation needed] In the United States it is sold under the brand name Noritate.[67]
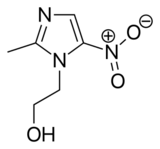
Synthesis
2-Methylimidazole (1) may be prepared via the Debus-Radziszewski imidazole synthesis, or from ethylenediamine and acetic acid, followed by treatment with lime, then Raney nickel. 2-Methylimidazole is nitrated to give 2-methyl-4(5)-nitroimidazole (2), which is in turn alkylated with ethylene oxide or 2-chloroethanol to give metronidazole (3):[68][69][70]
Research
Metronidazole is researched for its anti-inflammatory and immunomodulatory properties. Studies have shown that metronidazole can decrease the production of reactive oxygen species (ROS) and nitric oxide by activated immune cells, such as macrophages and neutrophils. Metronidazole's immunomodulatory properties are thought to be related to its ability to decrease the activation of nuclear factor-kappa B (NF-κB), a transcription factor that regulates the expression of pro-inflammatory cytokines, including chemokines, and adhesion molecules. Cytokines are small proteins that are secreted by immune cells and play a key role in the immune response.[71] Chemokines are a type of cytokines that act as chemoattractants, meaning they attract and guide immune cells to specific sites in the body where they are needed.[72] Cell adhesion molecules play an important role in the immune response by facilitating the interaction between immune cells and other cells in the body, such as endothelial cells, which form the lining of blood vessels.[73] By inhibiting NF-κB activation, metronidazole can reduce the production of pro-inflammatory cytokines, such as TNF-alpha, IL-6, and IL-1β.[74] Metronidazole has been studied in various immunological disorders, including inflammatory bowel disease, periodontitis, and rosacea. In these conditions, metronidazole has been suspected to have anti-inflammatory and immunomodulatory effects that could be beneficial in the treatment of these conditions.[75] Despite the success in treating rosacea with metronidazole,[76][77][78][79][80] the exact mechanism of why metronidazole in rosacea is efficient is not precisely known, i.e., which properties of metronidazole help treat rosacea: antibacterial or immunomodulatory or both, or other mechanism is involved.[81][82] Increased ROS production in rosacea is thought to contribute to the inflammatory process and skin damage, so metronidazole's ability to decrease ROS may explain the mechanism of action in this disease, but this remains speculation.[83][84]
Veterinary use
Metronidazole is used to treat infections of Giardia in dogs, cats, and other companion animals, but it does not reliably clear infection with this organism and is being supplanted by fenbendazole for this purpose in dogs and cats.[85] It is also used for the management of chronic inflammatory bowel disease in cats and dogs.[86] Another common usage is the treatment of systemic and/or gastrointestinal clostridial infections in horses. Metronidazole is used in the aquarium hobby to treat ornamental fish and as a broad-spectrum treatment for bacterial and protozoan infections in reptiles and amphibians. In general, the veterinary community may use metronidazole for any potentially susceptible anaerobic infection. The U.S. Food and Drug Administration (FDA) suggests it only be used when necessary because it has been shown to be carcinogenic in mice and rats, as well as to prevent antimicrobial resistance.[87][88]
References
- ↑ 1.0 1.1 1.2 "Metronidazole Use During Pregnancy". https://www.drugs.com/pregnancy/metronidazole.html.
- ↑ 2.0 2.1 "Metronidazole injection, solution". 16 January 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=3dc5f411-3312-4add-8198-161eed98132b.
- ↑ "Metronidazole tablet". 30 January 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=d7455566-16aa-488a-af2a-93b55a04c02f.
- ↑ "Metronidazole Vaginal Gel, 0.75%- metronidazole gel". 17 June 2023. https://dailymed.nlm.nih.gov/dailymed/drugInfo.cfm?setid=63ed1e7e-d264-4e35-af83-ec37fa5b06d1.
- ↑ 5.0 5.1 5.2 5.3 5.4 5.5 5.6 Cite error: Invalid
<ref>tag; no text was provided for refs namedMSR - ↑ 6.0 6.1 6.2 6.3 6.4 Cite error: Invalid
<ref>tag; no text was provided for refs namedMD - ↑ 7.0 7.1 7.2 7.3 7.4 7.5 7.6 7.7 "Metronidazole". The American Society of Health-System Pharmacists. https://www.drugs.com/monograph/metronidazole.html.
- ↑ 8.0 8.1 8.2 "Clinical Practice Guidelines for Clostridium difficile Infection in Adults and Children: 2017 Update by the Infectious Diseases Society of America (IDSA) and Society for Healthcare Epidemiology of America (SHEA)". Clinical Infectious Diseases 66 (7): e1–e48. March 2018. doi:10.1093/cid/cix1085. PMID 29462280.
- ↑ "Safety in Lactation: Metronidazole and tinidazole". https://www.sps.nhs.uk/articles/safety-in-lactation-metronidazole-and-tinidazole/.
- ↑ Drug discovery practices, processes, and perspectives. Hoboken, N.J.: John Wiley & Sons. 2013. p. 27. ISBN 9781118354469. https://books.google.com/books?id=E6Dv5XsXU-IC&pg=PA27.
- ↑ World Health Organization model list of essential medicines: 21st list 2019. Geneva: World Health Organization. 2019. WHO/MVP/EMP/IAU/2019.06. License: CC BY-NC-SA 3.0 IGO.
- ↑ "Trichomoniasis treatment in women". 28 July 2003. http://apps.who.int/rhl/rti_sti/gscom/en/.
- ↑ "The Top 300 of 2020". https://clincalc.com/DrugStats/Top300Drugs.aspx.
- ↑ "Metronidazole - Drug Usage Statistics". https://clincalc.com/DrugStats/Drugs/Metronidazole.
- ↑ "Metronidazole. A therapeutic review and update". Drugs 54 (5): 679–708. November 1997. doi:10.2165/00003495-199754050-00003. PMID 9360057.
- ↑ "Metronidazole is still the drug of choice for treatment of anaerobic infections". Clin Infect Dis 50 (Suppl 1): S16–23. January 2010. doi:10.1086/647939. PMID 20067388.
- ↑ 17.0 17.1 17.2 17.3 17.4 17.5 Australian Medicines Handbook (2013 ed.). Adelaide: The Australian Medicines Handbook Unit Trust. 2013. ISBN 978-0-9805790-9-3.
- ↑ "Clostridium difficile: diagnosis and treatment update". The Pharmaceutical Journal. Royal Pharmaceutical Society. 9 February 2017. https://www.pharmaceutical-journal.com/learning/cpd-article/clostridium-difficile-diagnosis-and-treatment-update/20202242.cpdarticle.
- ↑ Drugs in Palliative Care. OUP Oxford. 2012. p. 355. ISBN 9780191636103. https://books.google.com/books?id=mSzs_8ijZpgC&pg=PA355. Retrieved 29 August 2020.
- ↑ "Bacterial vaginosis: review of treatment options and potential clinical indications for therapy". Clinical Infectious Diseases 28 (Suppl 1): S57–S65. January 1999. doi:10.1086/514725. PMID 10028110.
- ↑ "Multicenter comparison of clotrimazole vaginal tablets, oral metronidazole, and vaginal suppositories containing sulfanilamide, aminacrine hydrochloride, and allantoin in the treatment of symptomatic trichomoniasis". Sexually Transmitted Diseases 24 (3): 156–160. March 1997. doi:10.1097/00007435-199703000-00006. PMID 9132982.
- ↑ "Drug Resistance in the Microaerophilic Parasite Giardia lamblia". Current Tropical Medicine Reports 2 (3): 128–135. September 2015. doi:10.1007/s40475-015-0051-1. PMID 26258002.
- ↑ "A comparison of vancomycin and metronidazole for the treatment of Clostridium difficile-associated diarrhea, stratified by disease severity". Clinical Infectious Diseases 45 (3): 302–307. August 2007. doi:10.1086/519265. PMID 17599306.
- ↑ Sherris medical microbiolog (Seventh ed.). New York. 12 January 2018. ISBN 978-1-259-85981-6. OCLC 1004770160.
- ↑ "Averting transmission: A pivotal target to manage amoebiasis". Chemical Biology & Drug Design 96 (2): 731–744. August 2020. doi:10.1111/cbdd.13699. PMID 32356312.
- ↑ "A randomised controlled trial of metronidazole for the prevention of preterm birth in women positive for cervicovaginal fetal fibronectin: the PREMET Study". BJOG 113 (1): 65–74. January 2006. doi:10.1111/j.1471-0528.2005.00788.x. PMID 16398774.
- ↑ D. Nori, J. M. Cain, B. S. Hilaris, W. B. Jones, J. L. Lewis: Metronidazole as a radiosensitizer and high-dose radiation in advanced vulvovaginal malignancies, a pilot study. In: Gynecologic Oncology. vol 16, issue 1, August 1983, S. 117–128, ISSN 0090-8258, PMID 6884824.
- ↑ J.R.Sarna, S.Furtado, A.K.Brownell: Neurologic complications of metronidazole. In: Can J Neurol Sci. vol 40, issue 6, November 2013, S. 768-776, PMID 24257215.
- ↑ J.Overgaard, H.S.Hansen, M.Overgaard, L.Bastholt, A.Berthelsen, L.Specht, et al.:A randomized double-blind phase III study of nimorazole as a hypoxic radiosensitizer of primary radiotherapy in supraglottic larynx and pharynx carcinoma. Results of the Danish Head and Neck Cancer Study (DAHANCA) In: Radiother Oncol vol 46, issue 2, May 1998, PMID 9510041.
- ↑ "Acneiform facial eruptions: a problem for young women". Canadian Family Physician 51 (4): 527–533. April 2005. PMID 15856972.
- ↑ Side Effects
- ↑ "Flagyl metronidazole tablets label". http://www.accessdata.fda.gov/drugsatfda_docs/label/2010/012623s061lbl.pdf.
- ↑ "Metronidazole ototoxicity--report of two cases". The Journal of Laryngology and Otology 113 (4): 355–357. April 1999. doi:10.1017/s0022215100143968. PMID 10474673.
- ↑ "Amebic abscess of the spleen complicated by metronidazole-induced neurotoxicity: case report". Clinical Infectious Diseases 19 (2): 346–348. August 1994. doi:10.1093/clinids/19.2.346. PMID 7986915.
- ↑ 35.0 35.1 "Pharmaceutical agents known to produce disulfiram-like reaction: effects on hepatic ethanol metabolism and brain monoamines". International Journal of Toxicology 26 (5): 423–432. 2007. doi:10.1080/10915810701583010. PMID 17963129.
- ↑ "The possibility of serotonin syndrome brought about by the use of metronidazole". Minerva Anestesiologica 74 (11): 679. November 2008. PMID 18971895.
- ↑ 37.0 37.1 37.2 37.3 "Metronidazole". Report on Carcinogens (Fourteenth ed.). National Toxicology Program (NTP). 2016. https://ntp.niehs.nih.gov/ntp/roc/content/profiles/metronidazole.pdf. Retrieved 9 February 2020.
- ↑ 38.0 38.1 "Metrogyl Metronidazole Product Information" (PDF). TGA eBusiness Services. Alphapharm Pty Limited. 8 May 2013. https://www.ebs.tga.gov.au/ebs/picmi/picmirepository.nsf/pdf?OpenAgent&id=CP-2010-PI-05077-3.
- ↑ "Is metronidazole carcinogenic?". Mutation Research 511 (2): 133–144. June 2002. doi:10.1016/S1383-5742(02)00007-8. PMID 12052431.
- ↑ "Cancer after exposure to metronidazole". Mayo Clinic Proceedings 63 (2): 147–153. February 1988. doi:10.1016/s0025-6196(12)64947-7. PMID 3339906.
- ↑ "Metronidazole (IARC Summary & Evaluation, Supplement7, 1987)". 3 March 1998. http://www.inchem.org/documents/iarc/suppl7/metronidazole.html.
- ↑ "Prenatal exposure to metronidazole and risk of childhood cancer: a retrospective cohort study of children younger than 5 years". Cancer 83 (7): 1461–1468. October 1998. doi:10.1002/(sici)1097-0142(19981001)83:7<1461::aid-cncr25>3.0.co;2-1. PMID 9762949.
- ↑ "Epidemiologic evaluation of pharmaceuticals with limited evidence of carcinogenicity". International Journal of Cancer 125 (9): 2173–2178. November 2009. doi:10.1002/ijc.24545. PMID 19585498.
- ↑ "Flagyl 375 U.S. Prescribing Information". Pfizer. http://www.pfizer.com/pfizer/download/uspi_flagyl_375.pdf.
- ↑ "Agents Classified by the IARC Monographs, Volumes 1–124". International Agency for Research on Cancer (IARC). 8 July 2019. https://monographs.iarc.fr/agents-classified-by-the-iarc/.
- ↑ "Outbreak of Stevens-Johnson syndrome/toxic epidermal necrolysis associated with mebendazole and metronidazole use among Filipino laborers in Taiwan". American Journal of Public Health 93 (3): 489–492. March 2003. doi:10.2105/ajph.93.3.489. PMID 12604501.
- ↑ "Sudden death due to metronidazole/ethanol interaction". The American Journal of Forensic Medicine and Pathology 17 (4): 343–346. December 1996. doi:10.1097/00000433-199612000-00013. PMID 8947362.
- ↑ "Effect of metronidazole on liver alcohol dehydrogenase". Biochemical Pharmacology 19 (10): 2805–2808. October 1970. doi:10.1016/0006-2952(70)90108-5. PMID 4320226.
- ↑ "Do ethanol and metronidazole interact to produce a disulfiram-like reaction?". The Annals of Pharmacotherapy 34 (2): 255–257. February 2000. doi:10.1345/aph.19118. PMID 10676835. "the authors of all the reports presumed the metronidazole-ethanol reaction to be an established pharmacologic fact. None provided evidence that could justify their conclusions".
- ↑ "Lack of disulfiram-like reaction with metronidazole and ethanol". The Annals of Pharmacotherapy 36 (6): 971–974. June 2002. doi:10.1345/1542-6270(2002)036<0971:lodlrw>2.0.co;2. PMID 12022894.
- ↑ "Drug Interactions: Cytochrome P450 Drug Interaction Table". Indiana University School of Medicine. 2007. http://medicine.iupui.edu/flockhart/table.htm.
- ↑ "Metronidazole reduces the expression of cytochrome P450 enzymes in HepaRG cells and cryopreserved human hepatocytes". Xenobiotica; the Fate of Foreign Compounds in Biological Systems 45 (5): 413–419. May 2015. doi:10.3109/00498254.2014.990948. PMID 25470432.
- ↑ "Potential CYP2C9-mediated drug-drug interactions in hospitalized type 2 diabetes mellitus patients treated with the sulphonylureas glibenclamide, glimepiride or glipizide". Journal of Internal Medicine 268 (4): 359–366. October 2010. doi:10.1111/j.1365-2796.2010.02257.x. PMID 20698928.
- ↑ 54.0 54.1 54.2 54.3 (in de) Austria-Codex. Vienna: Österreichischer Apothekerverlag. 2020. Anaerobex-Filmtabletten.
- ↑ "DNA and Chromosome Mechanics". Schaechter's Mechanisms of Microbial Disease. Hagerstown, MD: Lippincott Williams & Wilkins. 2007. p. 28. ISBN 978-0-7817-5342-5. https://books.google.com/books?id=1Zl70SLDU3oC&pg=PA28.
- ↑ "Pharmacokinetics and pharmacodynamics of the nitroimidazole antimicrobials". Clinical Pharmacokinetics 36 (5): 353–373. May 1999. doi:10.2165/00003088-199936050-00004. PMID 10384859.
- ↑ "Pharmacokinetics and bioavailability of metronidazole after tablets, suppositories and intravenous administration". Scandinavian Journal of Gastroenterology. Supplement 91: 45–60. 1984. PMID 6588489.
- ↑ Krakovka, Sascha; Ribacke, Ulf; Miyamoto, Yukiko; Eckmann, Lars; Svärd, Staffan (11 January 2022). "Characterization of Metronidazole-Resistant Giardia intestinalis Lines by Comparative Transcriptomics and Proteomics". Frontiers in Microbiology 13. doi:10.3389/fmicb.2022.834008. PMID 35222342.
- ↑ Alauzet, Corentine; Lozniewski, Alain; Marchandin, Hélène (11 February 2019). "Metronidazole resistance and nim genes in anaerobes: A review". Anaerobe 55: 40–53. doi:10.1016/j.anaerobe.2018.10.004. PMID 30316817. https://pubmed.ncbi.nlm.nih.gov/30316817/.
- ↑ Smith, A. (11 March 2018). "Metronidazole resistance: a hidden epidemic?". British Dental Journal 224 (6): 403–404. doi:10.1038/sj.bdj.2018.221. PMID 29545544. https://www.nature.com/articles/sj.bdj.2018.221.
- ↑ "Targeting the American market for medicines, ca. 1950s-1970s: ICI and Rhône-Poulenc compared". Bulletin of the History of Medicine 88 (4): 654–696. 29 December 2014. doi:10.1353/bhm.2014.0075. PMID 25557515.
- ↑ "G.D. SEARLE & CO. v. COMM | 88 T.C. 252 (1987) | 8otc2521326 | Leagle.com". https://www.leagle.com/decision/198734088otc2521326.
- ↑ "2003:Pfizer and Pharmacia Merger". https://www.pfizer.com/about/history/pfizer_pharmacia.
- ↑ "Effect of Evergreened Reformulations on Medicaid Expenditures and Patient Access from 2008 to 2016". Journal of Managed Care & Specialty Pharmacy 25 (7): 780–792. July 2019. doi:10.18553/jmcp.2019.18366. PMID 30799664.
- ↑ "Metrogyl ER". Medical Dialogues. https://medicaldialogues.in/partner/jbcpl/metrogyl-er-Metronidazole-Extended-release.
- ↑ "Metronidazole Brands". Medex. https://medex.com.bd/generics/751/metronidazole/brand-names.
- ↑ "Noritate (Metronidazole): Uses, Dosage, Side Effects, Interactions, Warning" (in en). https://www.rxlist.com/noritate-drug.htm.
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a13_661.
- ↑ "Ullmann's Encyclopedia of Industrial Chemistry". Ullmann's Encyclopedia of Industrial Chemistry. Weinheim: Wiley-VCH. doi:10.1002/14356007.a06_173.
- ↑ "Synthesis of metronidazole from ethylenediamine". Pharmaceutical Chemistry Journal 23 (10): 861–863. 1989. doi:10.1007/BF00764821.
- ↑ "Anti-inflammatory cytokines". Chest 117 (4): 1162–72. April 2000. doi:10.1378/chest.117.4.1162. PMID 10767254.
- ↑ "Chemokines". Arterioscler Thromb Vasc Biol 35 (11): e52–6. November 2015. doi:10.1161/ATVBAHA.115.306359. PMID 26490276.
- ↑ "Cell adhesion molecules--update". Vet Pathol 34 (1): 61–73. January 1997. doi:10.1177/030098589703400113. PMID 9150551.
- ↑ "Effect of metronidazole and modulation of cytokine production on human periodontal ligament cells". Int Immunopharmacol 10 (7): 744–50. July 2010. doi:10.1016/j.intimp.2010.04.004. PMID 20399284.
- ↑ "Pharmacological potential of new metronidazole/eugenol/dihydroeugenol hybrids against Trypanosoma cruzi in vitro and in vivo". Int Immunopharmacol 121: 110416. August 2023. doi:10.1016/j.intimp.2023.110416. PMID 37295025.
- ↑ "A systematic review to evaluate the efficacy of azelaic acid in the management of acne, rosacea, melasma and skin aging". J Cosmet Dermatol 22 (10): 2650–2662. October 2023. doi:10.1111/jocd.15923. PMID 37550898.
- ↑ "Ocular rosacea associated with transient monocular vision loss: resolution with oral metronidazole". Dermatol Online J 29 (3). June 2023. doi:10.5070/D329361439. PMID 37591279.
- ↑ "Maintenance of Remission after Oral Metronidazole Add-on Therapy in Rosacea Treatment: A Retrospective, Comparative Study". Ann Dermatol 34 (6): 451–460. December 2022. doi:10.5021/ad.22.093. PMID 36478427.
- ↑ "Perspectives on rosacea patient characteristics and quality of life using baseline data from a phase 3 clinical study conducted in Japan". J Dermatol 49 (12): 1221–1227. December 2022. doi:10.1111/1346-8138.16596. PMID 36177741.
- ↑ "Advances in pharmacotherapy for rosacea: what is the current state of the art?". Expert Opin Pharmacother 23 (16): 1845–1854. November 2022. doi:10.1080/14656566.2022.2142907. PMID 36330970.
- ↑ "Metronidazole Attenuates the Intensity of Inflammation in Experimental Autoimmune Uveitis". Folia Biol (Praha) 65 (5–6): 265–274. 2019. doi:10.14712/fb2019065050265. PMID 32362310.
- ↑ "Evaluation of immunosuppression induced by metronidazole in Balb/c mice and human peripheral blood lymphocytes". Int Immunopharmacol 8 (2): 341–50. February 2008. doi:10.1016/j.intimp.2007.10.018. PMID 18182250.
- ↑ "Reactive oxygen species and rosacea". Cutis 74 (3 Suppl): 17–20, 32–4. September 2004. PMID 15499754.
- ↑ "Scavenging properties of metronidazole on free oxygen radicals in a skin lipid model system". J Pharm Pharmacol 59 (8): 1125–30. August 2007. doi:10.1211/jpp.59.8.0010. PMID 17725855.
- ↑ "Efficacy of fenbendazole against giardiasis in dogs". American Journal of Veterinary Research 55 (7): 988–990. July 1994. doi:10.2460/ajvr.1994.55.07.988. PMID 7978640.
- ↑ "Advances in managing inflammatory bowel disease". DVM Newsmagazine. 1 October 2001. http://veterinarynews.dvm360.com/dvm/Gastroenterology/Advances-in-managing-inflammatory-bowel-disease/ArticleStandard/Article/detail/3000.
- ↑ Veterinary Drug Handbook (6th ed.). Wiley. 2008. ISBN 978-0-8138-2056-9.
- ↑ "Metronidazole". Drugs.com. https://www.drugs.com/pro/metronidazole.html.
External links
- "Metronidazole". Drug Information Portal. U.S. National Library of Medicine. https://druginfo.nlm.nih.gov/drugportal/name/Metronidazole.
- "Metronidazole and Tinidazole". Merck manuals. https://www.merckmanuals.com/professional/infectious-diseases/bacteria-and-antibacterial-drugs/metronidazole-and-tinidazole.
 |